PCI complications: dissection
|
Percutaneous coronary intervention Microchapters |
|
PCI Complications |
|---|
|
PCI in Specific Patients |
|
PCI in Specific Lesion Types |
|
PCI complications: dissection On the Web |
|
American Roentgen Ray Society Images of PCI complications: dissection |
|
Directions to Hospitals Treating Percutaneous coronary intervention |
|
Risk calculators and risk factors for PCI complications: dissection |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
A coronary artery dissection refers to a split or a tear in the wall of the artery which compresses or compromises the lumen of the artery reducing blood flow. Coronary artery dissection occurs rarely in the absence of percutaneous coronary intervention (PCI). The risk of reduced anterograde flow and lumen narrowing is a relatively (< 3%) uncommon complication of PCI. Significant residual dissections are present in approximately 1.7 % of patients undergoing PCI.[1] Intracoronary stent placement is used to stabilize the dissection.
Classification
The National Heart, Lung and Blood Institute (NHLBI) coronary dissection criteria assign according the severity of coronary dissection following PCI, with the prognostic implications of the coronary dissection depending on extension into the media and adventitia, its axial length, presence of contrast staining, and effect on anterograde coronary perfusion.
Difficulties can be present when assessing the angiographic residual lumen in the presence of coronary dissection due to the frame-to-frame lumen diameter changes using two-dimensional imaging; intravascular ultrasound (IVUS) may be more accurate strategy to provide the true circumference lumen dimensions.
In the NHLBI scheme, dissection is defined as an intraluminal filling defect or flap associated with a hazy, ground-glass appearance. This category is sub-classified using the NHLBI ([NHLBI|National Heart Lung and Blood Institute]]) system for grading dissection types:
Type A
Radiolucent areas within the coronary lumen during contrast injection, with minimal or no persistence of contrast after dye has cleared.
Type B
Parallel tracts or double lumen separated by a radiolucent area during contrast injection, with minimal or no persistence after dye has cleared.
Type C
Contrast outside the coronary lumen, with persistence of contrast in the area after dye has cleared.
Type D
Spiral luminal filling defects frequently with extensive contrast staining of the vessel.
Type E
New persistent filling defects that may be caused by thrombus.
Type F
These are non A – E dissection types that lead to impaired flow or total occlusion of the coronary artery.
Other associated definitions / terms include the following:
- Abrupt closure: Obstruction of contrast flow (TIMI 0 or 1) in a dilated segment with previously documented anterograde flow
- Ectasia: A lesion diameter greater than the reference diameter in one or more areas
- Luminal irregularities: Arterial contour that has a “sawtooth pattern” consisting of opacification but not fulfilling the criteria for dissection or intracoronary thrombus
- Intimal flap: A discrete filling defect in apparent continuity with the arterial wall
- Thrombus: Discrete, mobile angiographic filling defect with or without contrast staining
- Length:Measure end-to-end for type B through F dissections
- Staining: Persistence of contrast within the dissection after washout of contrast from the remaining portion of the vessel
- Perforation Localized: Extravasation of contrast confined to the pericardial space immediately surrounding the artery and not associated with clinical tamponade
- Nonlocalized perforation: Extravasation of contrast with a jet not localized to the pericardial space, potentially associated with clinical tamponade
- Side branch loss: TIMI 0, 1, or 2 flow in a side branch > 1.5 mm in diameter which previously had TIMI 3 flow
- Distal embolization: Migration of a filling defect or thrombus to distally occlude the target vessel or one of its branches
- Coronary spasm: Transient or permanent narrowing >50% when a <25% stenosis was previously noted
Pathophysiology
The two primary mechanisms accounting for disruption of the coronary vessel wall include barotrauma and guiding catheter dissections. Most coronary dissections occur as a result of percutaneous coronary intervention, but they may also occur either as an extension of an aortic dissection into the right coronary artery, or in the setting of coronary bypass grafting. Spontaneous coronary dissection in the coronary artery is itself rare.
Histopathological Findings
-
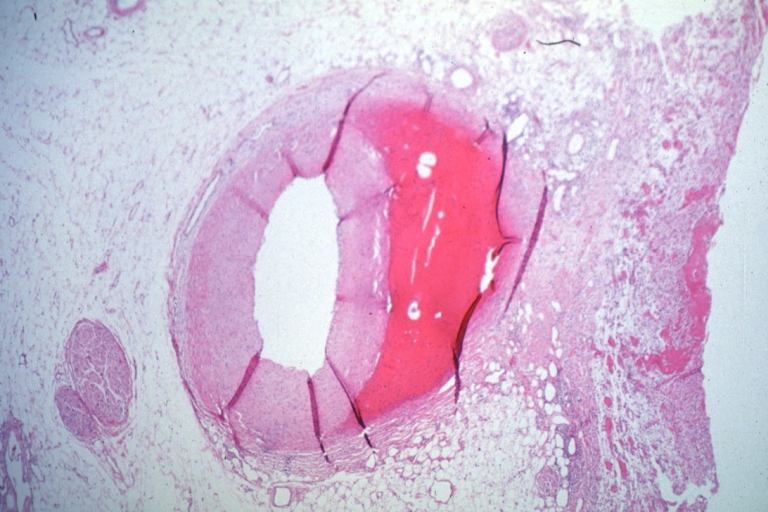
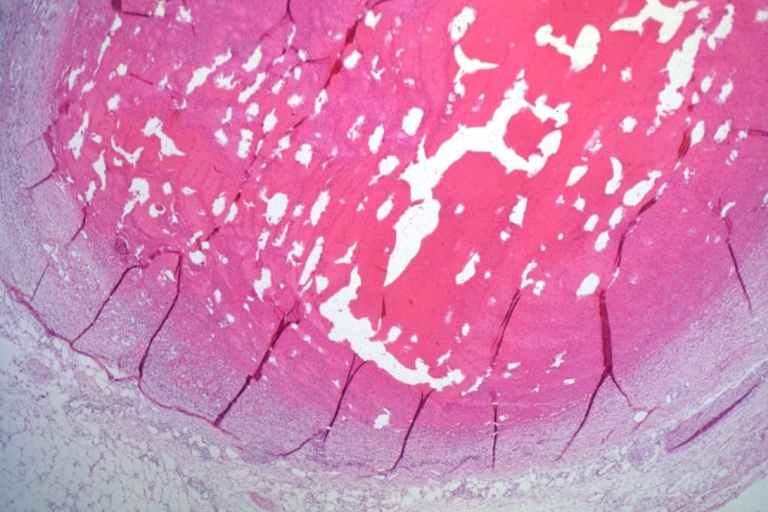
Coronary artery: Dissection Secondary to Trauma: Micro low mag H&E large adventitial hemorrhage this is a diagonal branch of the left anterior descending artery caused massive infarct
-
Coronary artery: Dissection Secondary to Trauma: Micro low mag H&E completely occluded LAD
Spontaneous coronary artery dissection (SCAD)
The majority of dissections occur following percutaneous coronary intervention (PCI). However, although rare, coronary dissections can occur spontaneously. The literature is sparse with only approximately 300 documented cases of spontaneous dissection.[2]
Epidemiology and Demographics
The incidence of SCAD in angiographic series is between 0.28 and 1.1%, and most 60% of the time the dissection occurs in the left anterior descending artery (LAD). [2] 80% of SCAD cases occur in young women, with those taking birth control medication or in the peripartum period at particular risk. [3]
Risk Factors
It has been hypothesized that the strain of childbirth and the release of systemic factors such as relaxin underly the increased risk. Other factors that may be involved include changes in hormones and autoimmune status as well as hemodynamic stress[4]. SCAD has also been found in patients with atherosclerosis, connective tissue disorders. [5] and bicuspid aortic stenosis[6]
Natural History, Complications and Prognosis
The fact that the diagnosis was made so often in the past on autopsy speaks to the poor clinical outcomes that have been associated with the condition. Outcomes in the modern era of stent placement and improved antithrombins may be improved, but solid data are lacking.
Diagnosis
The symptoms of SCAD mimic other acute coronary syndromes. In the past, this disorder was often diagnosed only at the time of autopsy.[7] At present, however, angiography is most often used to diagnose SCAD, although intravascular ultrasound and CT angiography are also used.[2]
Treatment
Coronary stent placement, surgery and anticoagulation would appear to be reasonable treatment modalities. [8]. Given the rare nature of the disease, randomized trial data is obviously lacking. Most intra-procedural dissections can be treated promptly with stenting.
References
- ↑ Laskey WK, Williams DO, Vlachos HA; et al. (2001). "Changes in the practice of percutaneous coronary intervention: a comparison of enrollment waves in the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry". Am. J. Cardiol. 87 (8): 964–9, A3–4. PMID 11305987. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 2.2 Kamran M, Guptan A, Bogal M (2008). "Spontaneous coronary artery dissection: case series and review". J Invasive Cardiol. 20 (10): 553–9. PMID 18830003. Unknown parameter
|month=ignored (help) - ↑ Narasimhan, S (2004). "Spontaneous coronary artery dissection (SCAD)" (PDF). IJTCVS. 20 (4): 189–91. doi:10.1007/s12055-004-0084-x.
- ↑ Van den Branden BJ, Bruggeling WA, Corbeij HM, Dunselman PH (2008). "Spontaneous coronary artery dissection in the postpartum period". Neth Heart J. 16 (12): 412–4. PMC 2612109. PMID 19127318. Unknown parameter
|month=ignored (help) - ↑ Tanis W, Stella PR, Kirkels JH, Pijlman AH, Peters RH, de Man FH (2008). "Spontaneous coronary artery dissection: current insights and therapy". Neth Heart J. 16 (10): 344–9. PMC 2570766. PMID 18958258. Unknown parameter
|month=ignored (help) - ↑ Archives of cardiovascular diseases. Volume 102, n° 12 pages 857-858 (décembre 2009).
- ↑ Narasimhan, S (2004). "Spontaneous coronary artery dissection (SCAD)" (PDF). IJTCVS. 20 (4): 189–91. doi:10.1007/s12055-004-0084-x.
- ↑ Narasimhan, S (2004). "Spontaneous coronary artery dissection (SCAD)" (PDF). IJTCVS. 20 (4): 189–91. doi:10.1007/s12055-004-0084-x.