Niacin (extended-release tablet): Difference between revisions
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
|authorTag={{AP}} | |authorTag={{AP}} | ||
|genericName=Nicotinic acid | |genericName=Nicotinic acid | ||
|drugClass=[[antihyperlipidemic]], [[nutriceutical]], [[nutritive agent]], [[Vitamin B]] | |aOrAn=an | ||
|drugClass=[[antihyperlipidemic]], [[nutriceutical]], [[nutritive agent]], [[Vitamin B]] | |||
|indicationType=treatment | |indicationType=treatment | ||
|indication=[[hyperlipidemia]], prevention of recurrence of [[myocardial infarction]], reduce [[atheroesclerotic plaque]] in [[CAD]] and [[hypertriglyceridemia]] in patients with risk for [[pancreatitis]] | |indication=[[hyperlipidemia]], prevention of recurrence of [[myocardial infarction]], reduce [[atheroesclerotic plaque]] in [[CAD]] and [[hypertriglyceridemia]] in patients with risk for [[pancreatitis]] | ||
|adverseReactions=[[flushing]], [[diarrhea]], [[nausea]], [[vomiting]], increased [[cough]], and [[pruritus]] | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| Line 11: | Line 13: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Niacin in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Niacin in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Niacin in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Niacin in pediatric patients. | ||
|contraindications=Niacin extended-release tablet is contraindicated in the following conditions: | |||
*Active [[liver disease]] or unexplained persistent elevations in [[hepatic transaminases]]. | |||
*Patients with active [[peptic ulcer disease]] | |||
*Patients with [[arterial bleeding]] | |||
*[[Hypersensitivity]] to niacin or any component of this medication. | |||
|alcohol=Alcohol-Niacin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Niacin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:52, 6 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Alberto Plate [5]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Niacin (extended-release tablet) is an antihyperlipidemic, nutriceutical, nutritive agent, Vitamin B that is FDA approved for the treatment of hyperlipidemia, prevention of recurrence of myocardial infarction, reduce atheroesclerotic plaque in CAD and hypertriglyceridemia in patients with risk for pancreatitis. Common adverse reactions include flushing, diarrhea, nausea, vomiting, increased cough, and pruritus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Niacin (extended-release tablet) FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Niacin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Niacin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Niacin (extended-release tablet) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Niacin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Niacin in pediatric patients.
Contraindications
Niacin extended-release tablet is contraindicated in the following conditions:
- Active liver disease or unexplained persistent elevations in hepatic transaminases.
- Patients with active peptic ulcer disease
- Patients with arterial bleeding
- Hypersensitivity to niacin or any component of this medication.
Warnings
There is limited information regarding Niacin (extended-release tablet) Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Niacin (extended-release tablet) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Niacin (extended-release tablet) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Niacin (extended-release tablet) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Niacin (extended-release tablet) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Niacin (extended-release tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Niacin (extended-release tablet) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Niacin (extended-release tablet) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Niacin (extended-release tablet) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Niacin (extended-release tablet) in geriatric settings.
Gender
There is no FDA guidance on the use of Niacin (extended-release tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Niacin (extended-release tablet) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Niacin (extended-release tablet) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Niacin (extended-release tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Niacin (extended-release tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Niacin (extended-release tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Niacin (extended-release tablet) Administration in the drug label.
Monitoring
There is limited information regarding Niacin (extended-release tablet) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Niacin (extended-release tablet) and IV administrations.
Overdosage
There is limited information regarding Niacin (extended-release tablet) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Niacin (extended-release tablet) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Niacin (extended-release tablet) Mechanism of Action in the drug label.
Structure
There is limited information regarding Niacin (extended-release tablet) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Niacin (extended-release tablet) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Niacin (extended-release tablet) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Niacin (extended-release tablet) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Niacin (extended-release tablet) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Niacin (extended-release tablet) How Supplied in the drug label.
Storage
There is limited information regarding Niacin (extended-release tablet) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Niacin (extended-release tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Niacin (extended-release tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Niacin (extended-release tablet) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Niacin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Niacin (extended-release tablet) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Niacin (extended-release tablet) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [6]
Overview
Niacin, also known as nicotinic acid or vitamin B3, is a water-soluble vitamin discovered by Conrad Elvehjem in 1937. Its derivatives, NADH, NAD, NAD+, and NADP play essential roles in energy metabolism in the living cell and DNA repair (an enzymatic process in a living cell). [1] The designation vitamin B3 also includes the corresponding amide nicotinamide (or "niacinamide"), whose chemical formula is C6H6N2O.
Other functions of niacin include removing toxic chemicals from the body,[2] and assisting in the production of steroid hormones made by the adrenal gland, such as sex hormones and stress-related hormones.
History
Niacin was first discovered from the oxidation of nicotine to form nicotinic acid. When the properties of nicotinic acid were discovered, it was thought prudent to choose a name to dissociate it from nicotine, in order to avoid the perception that vitamins or niacin-rich food contains nicotine. The resulting name 'niacin' was derived from nicotinic acid + vitamin.
Niacin is also referred to as Vitamin B3 because it was the third of the B vitamins to be discovered. It has historically been referred to as "vitamin PP", a name derived from the term "pellagra-preventing factor".
Dietary needs
The recommended daily allowance of niacin is 2-12 mg a day for children, 14 mg a day for women, 16 mg a day for men, and 18 mg a day for pregnant or breast-feeding women.[3]
Severe deficiency of niacin in the diet causes the disease pellagra, whereas mild deficiency slows down the metabolism, causing decreased tolerance to cold.
Dietary niacin deficiency tends to occur only in areas where people eat corn (maize), the only grain low in niacin, as a staple food, and that do not use lime during meal/flour production. Alkali lime releases the tryptophan from the corn in a process called nixtamalization so that it can be absorbed in the intestine, and converted to niacin.[2]
Pharmacological uses
Niacin, when taken in large doses, blocks the breakdown of fats in adipose tissue, thus altering blood lipid levels. Niacin is used in the treatment of hyperlipidemia because it reduces very-low-density lipoprotein (VLDL), a precursor of low-density lipoprotein (LDL) or "bad" cholesterol. Because niacin blocks breakdown of fats, it causes a decrease in free fatty acids in the blood and, as a consequence, decreased secretion of VLDL and cholesterol by the liver.[4]
By lowering VLDL levels, niacin also increases the level of high-density lipoprotein (HDL) or "good" cholesterol in blood, and therefore it is sometimes prescribed for patients with low HDL, who are also at high risk of a heart attack.[5][6] An extended release formulation of niacin for this indication is marketed by Abbott Laboratories under the trade name Niaspan.
Niacin is sometimes consumed in large quantities by people who wish to fool drug screening tests, particularly for lipid-soluble drugs such as marijuana.[7] It is believed to "promote metabolism" of the drug and cause it to be "flushed out." Scientific studies have shown it does not affect drug screenings, but can pose a risk of overdose, causing arrhythmias, metabolic acidosis, hyperglycemia, and other serious problems (see below).
In October 2008, Merck expanded the THRIVE Trial from 20,000 to 25,000 patients in order to expedite a second bid for FDA approval of its experimental cholesterol drug, MK-0524A. MK-0524A is a combination of niacin and laropiprant, which is aimed at limiting facial flushing associated with niacin. In April 2008 the FDA decided to withhold approval for the experimental drug, deciding to wait until the results of the THRIVE Trial could be analyzed.[8]
Toxicity
People taking pharmacological doses of niacin (1.5 - 6 g per day) often experience a syndrome of side-effects that can include one or more of the following:[9]
- dermatological complaints
- facial flushing and itching
- dry skin
- skin rashes including acanthosis nigricans
- gastrointestinal complaints
- dyspepsia (indigestion)
- liver toxicity
- hyperglycemia
- cardiac arrhythmias
- birth defects
Facial flushing is the most commonly-reported side-effect.[10] It lasts for about 15 to 30 minutes, and is sometimes accompanied by a prickly or itching sensation. This effect is mediated by prostaglandins and can be blocked by taking 300 mg of aspirin half an hour before taking niacin, or by taking one tablet of ibuprofen per day. Taking the niacin with meals also helps reduce this side-effect. After 1 to 2 weeks of a stable dose, most patients no longer flush. Slow- or "sustained"-release forms of niacin have been developed to lessen these side-effects.[11][12] [13] One study showed the incidence of flushing was 4.5x lower (1.9 vs. 8.6 episodes in the first month) with a sustained-release formulation.[14]
Doses above 2 g per day have been associated with liver damage, particularly with slow-release formulations. [15]
High-dose niacin may also elevate blood sugar, thereby worsening diabetes mellitus.[16] Hyperuricemia is another side-effect of taking high-dose niacin; thus niacin may worsen gout.
Niacin at doses used in lowering cholesterol has been associated with birth defects in laboratory animals and should not be taken by pregnant women.[17]
Niacin at extremely high doses can have life-threatening acute toxic reactions. One patient suffered vomiting after taking eleven 500-milligram niacin tablets over 36 hours, and another was unresponsive for several minutes after taking five 500-milligram tablets over two days.[18][19] Extremely high doses of niacin can also cause niacin maculopathy, a thickening of the macula and retina which leads to blurred vision and blindness.[20]
Inositol hexanicotinate
One popular form of dietary supplement is inositol hexanicotinate, usually sold as "flush-free" or "no-flush" niacin (although those terms are also used for regular sustained-release.) While this form of niacin does not cause the flushing associated with the nicotinic acid form, it is not clear whether it is pharmacologically equivalent in its positive effect.[21]
Biosynthesis
The liver can synthesize niacin from the essential amino acid tryptophan (see below), but the synthesis is extremely inefficient; 60 mg of tryptophan are required to make one milligram of niacin.[22]
The 5-membered aromatic heterocycle of the essential amino acid, tryptophan, is cleaved and rearranged with the alpha amino group of tryptophan into the 6-membered aromatic heterocycle of niacin by the following reaction:
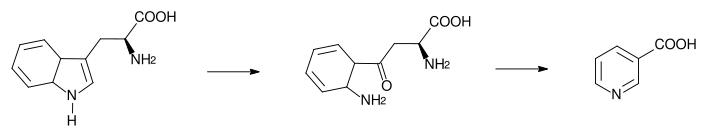
Biosynthesis: Tryptophan → kynurenine → niacin
Receptor
The receptor for niacin is a G-protein coupled receptor called HM74A.[23] It couples to Gi[24].
Food sources
| Animal products: | Fruits and vegetables: | Seeds: | Fungi: |
|---|---|---|---|
|
References
- ↑ Northwestern University Nutrition
- ↑ 2.0 2.1 Vitamin B3 University of Maryland Medical Center.
- ↑ Template:Pauling
- ↑ T. Katzung, Basic and Clinical Pharmacology, 9th ed. p. 570.
- ↑ Postgraduate Medicine
- ↑ Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8(6):1245-1255.
- ↑ Niacin abuse in the attempt to alter urine drug tests. Pharmacist's Letter/Prescriber's Letter 2007;23(6):230606.
- ↑ http://biz.yahoo.com/ap/081017/merck_cholesterol_study.html?.v=2
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.991.
- ↑ NIH Medline Plus: Niacin. http://www.nlm.nih.gov/medlineplus/ency/article/002409.htm.
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.991.
- ↑ T. Katzung, Basic and Clinical Pharmacology, 9th ed. p. 570.
- ↑ Options for therapeutic intervention: How effective are the different agents? European Heart Journal Supplements Vol 8 Suppl F Pp. F47-F53 [1]
- ↑ Chapman M, Assmann G, Fruchart J, Sheperd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid - a position paper developed by the European Consensus Panel on HDL-C. Cur Med Res Opin. 2004 Aug;20(8):1253-68. PMID 15324528
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.992.
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.991.
- ↑ J.G. Hardman et al., eds., Goodman and Gilman's Pharmacological Basis of Therapeutics, 10th ed., p.992.
- ↑ Hazards: Niacin to Pass a Drug Test Can Have Dangerous Results, By ERIC NAGOURNEY, New York Times, April 17, 2007[2]
- ↑ Mittal MK, Florin T, Perrone J, Delgado JH, Osterhoudt KC. Toxicity From the Use of Niacin to Beat Urine Drug Screening. Ann Emerg Med. 2007 Apr 4. PMID 17418450[3]
- ↑ JD Gass, Nictonic Acid Maculopathy, Am. J. Opthamology, 1973;76:500-10
- ↑ No-Flush Niacin for the Treatment of Hyperlipidemia
- ↑ Oxidization Reactions of Niacin from the Linus Pauling Institute at Oregon State University Linus Pauling Institute.
- ↑ medscape.com - The Metabolic Syndrome: Etiology, Controversies, and Emerging ...
- ↑ Variations in human HM74 (GPR109B) and HM74A (GPR109A) niacin receptors Christian Zellner 1 *, Clive R. Pullinger 1, Bradley E. Aouizerat 2, Philip H. Frost 1, Pui-Yan Kwok 1, Mary J. Malloy 1, John P. Kane
External links
Template:Peripheral vasodilators Template:Lipid modifying agents