Nasopharyngeal angiofibroma
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mahda Alihashemi M.D. [2] [3] [4] [5] [6] [7] [8]
Synonyms and keywords: Juvenile nasopharyngeal angiofibroma (JNA); angiofibroma of the nasopharynx, nasopharyngeal fibroma, bleeding fibroma of adolescence, fibroangioma
Overview
Historical Perspective
- Nasopharyngeal angiofibroma was first described by Hippocrates, a Greek physician, in the 5th century BC.[1]
- In 1940, Friedberg was the first to use the term angiofibroma.
Classification
Nasopharyngeal angiofibroma may be classified according to Fisch classification into 4 groups: [2]
- Type I: Sphenopalatine foramen, nasopharynx, and nasal cavity are involved without bone destruction.
- Type II: Nasal sinuses or the pterygomaxillary fossa are involved with bone destruction.
- Type IIIa: Infratemporal fossa or orbit are involved without intracranial involvement
- Type IIIb: Infratemporal fossa or orbit are involved with intracranial extradural commitment.
- Type IVa: Intracranial extradural and/or intradural are involved, but optic nerve, sella, or cavernous sinus are not involved.
- Type IVb: Intracranial extradural and intradura, optic nerve, sella, and/or cavernous sinusare are involved
.
Pathophysiology
Nasopharyngeal angiofibroma is a histologically benign tumor. This tumor is located in the posterolateral wall of the nasal cavity. Nasopharyngeal angiofibroma may involve sphenoid sinuses, maxillary ethmoid, pterygoid plate , orbit, base of the skull and extradural. In rare cases, nasopharyngeal angiofibroma may involve pituitary, cavernous sinus and/or optic chiasm and anterior fossa. [3]
- Genetic alterations associated with the development of nasopharyngeal angiofibroma include:[4]
- Overexpression PDGF-B
- Overexpression bFGF
- Overexpression bFGF
- Deletion of chromosome 17
- Tumor suppressor gene p53
- Overexpression of Her-2/neu oncogene
- On gross pathology, characteristic findings of nasopharyngeal angiofibroma include:
- Bilobed or dumbbell-shaped with average size 3-5 cm [5]
- Tan to purple-red, rubbery-firm unencapsulated polypoid fibrous mass
- Bleeding on biopsy
- Spongy cut surface
- Sessile or pedunculated tumor
- On microscopic histopathological analysis, characteristic findings of nasopharyngeal angiofibroma include:
- Fibroblastic cells with plump (near cuboidal) nuclei
- Fibrous stroma
- Abundant capillaries
- Multinucleated stromal cells
Causes
The cause of Nasopharyngeal angiofibroma has not been identified, but it may be caused by hormonal effect. [6] Nasopharyngeal angiofibroma express higher levels of vascular endothelial growth factor (VEGF) and hormone receptors.To review risk factors for the development of Nasopharyngeal angiofibroma, click here.
Differentiating Nasopharyngeal angiofibroma from Other Diseases
- Nasopharyngeal angiofibroma must be differentiated from other diseases that cause epistaxis, unilateral nasal obstruction, and rhinorrhea, such as:
- Antro-choanal polyp
- Rhinosporidiosis
- Chordoma
- Nasopharanageal cyst
- Pyogenic granuloma
[Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as [differential dx1], [differential dx2], and [differential dx3].
OR
[Disease name] must be differentiated from [[differential dx1], [differential dx2], and [differential dx3].
Epidemiology and Demographics
The incidence/prevalence of [disease name] is approximately [number range] per 100,000 individuals worldwide.
OR
In [year], the incidence/prevalence of [disease name] was estimated to be [number range] cases per 100,000 individuals worldwide.
OR
In [year], the incidence of [disease name] is approximately [number range] per 100,000 individuals with a case-fatality rate of [number range]%.
Patients of all age groups may develop [disease name].
OR
The incidence of [disease name] increases with age; the median age at diagnosis is [#] years.
OR
[Disease name] commonly affects individuals younger than/older than [number of years] years of age.
OR
[Chronic disease name] is usually first diagnosed among [age group].
OR
[Acute disease name] commonly affects [age group].
There is no racial predilection to [disease name].
OR
[Disease name] usually affects individuals of the [race 1] race. [Race 2] individuals are less likely to develop [disease name].
[Disease name] affects men and women equally.
OR
[Gender 1] are more commonly affected by [disease name] than [gender 2]. The [gender 1] to [gender 2] ratio is approximately [number > 1] to 1.
The majority of [disease name] cases are reported in [geographical region].
OR
[Disease name] is a common/rare disease that tends to affect [patient population 1] and [patient population 2].
Risk Factors
There are no established risk factors for [disease name].
OR
The most potent risk factor in the development of [disease name] is [risk factor 1]. Other risk factors include [risk factor 2], [risk factor 3], and [risk factor 4].
OR
Common risk factors in the development of [disease name] include [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
OR
Common risk factors in the development of [disease name] may be occupational, environmental, genetic, and viral.
Screening
There is insufficient evidence to recommend routine screening for [disease/malignancy].
OR
According to the [guideline name], screening for [disease name] is not recommended.
OR
According to the [guideline name], screening for [disease name] by [test 1] is recommended every [duration] among patients with [condition 1], [condition 2], and [condition 3].
Natural History, Complications, and Prognosis
If left untreated, [#]% of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
OR
Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
OR
Prognosis is generally excellent/good/poor, and the 1/5/10-year mortality/survival rate of patients with [disease name] is approximately [#]%.
Diagnosis
Diagnostic Study of Choice
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: [criterion 1], [criterion 2], [criterion 3], and [criterion 4].
OR
The diagnosis of [disease name] is based on the [criteria name] criteria, which include [criterion 1], [criterion 2], and [criterion 3].
OR
The diagnosis of [disease name] is based on the [definition name] definition, which includes [criterion 1], [criterion 2], and [criterion 3].
OR
There are no established criteria for the diagnosis of [disease name].
History and Symptoms
The classic triad of epistaxis, unilateral nasal obstruction, and a mass in the nasopharynx suggests a diagnosis of nasopharyngeal angiofibroma and is supplemented by imaging11 12 13 14 15. A biopsy is recommended only in cases of diagnostic uncertainty4. Examinations such as computed tomography, nuclear magnetic resonance and even nasal endoscopy can clearly establish the extent of the tumor, its pattern of spread, and consequently, surgical planning
OR
The hallmark of Nasopharyngeal angiofibroma is the classic triad of epistaxis, unilateral nasal obstruction and a mass in the nasopharynx . The most common symptoms of Nasopharyngeal angiofibroma include nasal obstruction and epistaxis and headache. Common symptoms of [disease] include [symptom 1], [symptom 2], and [symptom 3]. Less common symptoms of Nasopharyngeal angiofibroma include conductive hearing loss, diplopia, proptosis, anosmia, recurrent ottits media and eye pain. [7]
Physical Examination
Physical examination of patients with Nasopharyngeal angiofibroma is usually remarkable for nasal mass and rhinorrhea.[7]
Rare physical examination include:
- Orbital mass
- Proptosis
- Zygomatic swelling
- Trismus
- Decreasing vision
OR
Common physical examination findings of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
The presence of [finding(s)] on physical examination is diagnostic of [disease name].
OR
The presence of [finding(s)] on physical examination is highly suggestive of [disease name].
Laboratory Findings
An elevated/reduced concentration of serum/blood/urinary/CSF/other [lab test] is diagnostic of [disease name].
OR
Laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
OR
[Test] is usually normal among patients with [disease name].
OR
Some patients with [disease name] may have elevated/reduced concentration of [test], which is usually suggestive of [progression/complication].
OR
There are no diagnostic laboratory findings associated with [disease name].
Electrocardiogram
There are no ECG findings associated with [disease name].
OR
An ECG may be helpful in the diagnosis of [disease name]. Findings on an ECG suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
X-ray
There are no x-ray findings associated with [disease name].
OR
An x-ray may be helpful in the diagnosis of [disease name]. Findings on an x-ray suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no x-ray findings associated with [disease name]. However, an x-ray may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Echocardiography or Ultrasound
There are no echocardiography/ultrasound findings associated with [disease name].
OR
Echocardiography/ultrasound may be helpful in the diagnosis of [disease name]. Findings on an echocardiography/ultrasound suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no echocardiography/ultrasound findings associated with [disease name]. However, an echocardiography/ultrasound may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
CT scan
There are no CT scan findings associated with [disease name].
OR
[Location] CT scan may be helpful in the diagnosis of [disease name]. Findings on CT scan suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no CT scan findings associated with [disease name]. However, a CT scan may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
MRI
There are no MRI findings associated with [disease name].
OR
[Location] MRI may be helpful in the diagnosis of [disease name]. Findings on MRI suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
There are no MRI findings associated with [disease name]. However, a MRI may be helpful in the diagnosis of complications of [disease name], which include [complication 1], [complication 2], and [complication 3].
Other Imaging Findings
There are no other imaging findings associated with [disease name].
OR
[Imaging modality] may be helpful in the diagnosis of [disease name]. Findings on an [imaging modality] suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
There are no other diagnostic studies associated with [disease name].
OR
[Diagnostic study] may be helpful in the diagnosis of [disease name]. Findings suggestive of/diagnostic of [disease name] include [finding 1], [finding 2], and [finding 3].
OR
Other diagnostic studies for [disease name] include [diagnostic study 1], which demonstrates [finding 1], [finding 2], and [finding 3], and [diagnostic study 2], which demonstrates [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
There is no treatment for [disease name]; the mainstay of therapy is supportive care.
OR
Supportive therapy for [disease name] includes [therapy 1], [therapy 2], and [therapy 3].
OR
The majority of cases of [disease name] are self-limited and require only supportive care.
OR
[Disease name] is a medical emergency and requires prompt treatment.
OR
The mainstay of treatment for [disease name] is [therapy].
OR The optimal therapy for [malignancy name] depends on the stage at diagnosis.
OR
[Therapy] is recommended among all patients who develop [disease name].
OR
Pharmacologic medical therapy is recommended among patients with [disease subclass 1], [disease subclass 2], and [disease subclass 3].
OR
Pharmacologic medical therapies for [disease name] include (either) [therapy 1], [therapy 2], and/or [therapy 3].
OR
Empiric therapy for [disease name] depends on [disease factor 1] and [disease factor 2].
OR
Patients with [disease subclass 1] are treated with [therapy 1], whereas patients with [disease subclass 2] are treated with [therapy 2].
Surgery
Surgical intervention is not recommended for the management of [disease name].
OR
Surgery is not the first-line treatment option for patients with [disease name]. Surgery is usually reserved for patients with either [indication 1], [indication 2], and [indication 3]
OR
The mainstay of treatment for [disease name] is medical therapy. Surgery is usually reserved for patients with either [indication 1], [indication 2], and/or [indication 3].
OR
The feasibility of surgery depends on the stage of [malignancy] at diagnosis.
OR
Surgery is the mainstay of treatment for [disease or malignancy].
Primary Prevention
There are no established measures for the primary prevention of Nasopharyngeal angiofibroma.
Secondary Prevention
There are no established measures for the secondary prevention of [disease name].[8]
OR
Effective measures for the secondary prevention of [disease name] include [strategy 1], [strategy 2], and [strategy 3].
References
- ↑ Moorthy PN, Ranganatha Reddy B, Qaiyum HA, Madhira S, Kolloju S (October 2010). "Management of juvenile nasopharyngeal angiofibroma: a five year retrospective study". Indian J Otolaryngol Head Neck Surg. 62 (4): 390–4. doi:10.1007/s12070-010-0097-2. PMC 3266082. PMID 22319699.
- ↑ Martins MB, de Lima FV, Mendonça CA, de Jesus EP, Santos AC, Barreto VM, Santos RC (January 2013). "Nasopharyngeal angiofibroma: Our experience and literature review". Int Arch Otorhinolaryngol. 17 (1): 14–9. doi:10.7162/S1809-97772013000100003. PMC 4423317. PMID 25991988.
- ↑ Pryor SG, Moore EJ, Kasperbauer JL (July 2005). "Endoscopic versus traditional approaches for excision of juvenile nasopharyngeal angiofibroma". Laryngoscope. 115 (7): 1201–7. doi:10.1097/01.MLG.0000162655.96247.66. PMID 15995507.
- ↑
- ↑ McKnight CD, Parmar HA, Watcharotone K, Mukherji SK (2017). "Reassessing the Anatomic Origin of the Juvenile Nasopharyngeal Angiofibroma". J Comput Assist Tomogr. 41 (4): 559–564. doi:10.1097/RCT.0000000000000566. PMID 28632604.
- ↑ Liu Z, Wang J, Wang H, Wang D, Hu L, Liu Q, Sun X (January 2015). "Hormonal receptors and vascular endothelial growth factor in juvenile nasopharyngeal angiofibroma: immunohistochemical and tissue microarray analysis". Acta Otolaryngol. 135 (1): 51–7. doi:10.3109/00016489.2014.952774. PMID 25384380.
- ↑ 7.0 7.1 Enepekides DJ (December 2004). "Recent advances in the treatment of juvenile angiofibroma". Curr Opin Otolaryngol Head Neck Surg. 12 (6): 495–9. PMID 15548906.
- ↑ Blount A, Riley KO, Woodworth BA (August 2011). "Juvenile nasopharyngeal angiofibroma". Otolaryngol. Clin. North Am. 44 (4): 989–1004, ix. doi:10.1016/j.otc.2011.06.003. PMID 21819885.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [9] Associate Editor(s)-in-Chief: Maria Fernanda Villarreal, M.D. [10]
Synonyms and keywords: Juvenile nasopharyngeal angiofibroma; angiofibroma of the nasopharynx; JNA
Overview
Nasopharyngeal angiofibroma (also called juvenile nasopharyngeal angiofibroma) is a locally aggressive, benign vascular neoplasm that grows in the pterygopalatine fossa. The most common symptoms of nasopharyngeal angiofibroma include one-sided nasal obstruction and recurrent bleeding. Nasopharyngeal angiofibroma may be classified according to Radkowski Classification System (severity) into 3 categories: I, II, and III. The majority of nasopharyngeal angiofibromas are irrigated by the external carotid artery. Genetic alterations associated with the development of nasopharyngeal angiofibroma include: overexpression of PDGF-B, bFGF, VEGF. Nasopharyngeal angiofibroma accounts for 0.05% of all head and neck tumors. Nasopharyngeal angiofibromas are more commonly observed among children and adolescents. Males are more commonly affected with nasopharyngeal angiofibroma than females. Common risk factors in the development of nasopharyngeal angiofibroma, include: presence of tumor in the pterygoid fossa and young age. Physical examination may be remarkable for smooth submucosal mass in the posterior nasal cavity. Computed tomography is the imaging modality of choice. On conventional radiography, findings of nasopharyngeal angiofibroma include: visualization of a nasopharyngeal mass, opacification of the sphenoid sinus, and anterior bowing of the posterior wall of the maxillary antrum. Surgery is the mainstay of therapy for nasopharyngeal angiofibroma. Surgical approach for nasopharyngeal angiofibroma will usually depend on the stage. The treatment of choice for early stage for nasopharyngeal angiofibroma is intranasal endoscopic surgery ( e.g. lateral rhinotomy). The treatment of choice for late stage nasopharyngeal angiofibroma include the infratemporal fossa approach, and the mid-facial degloving approach. Common complications of nasopharyngeal angiofibroma include transient blindness, optic nerve damage, and low-grade consumption coagulopathy.
Historical Perspective
- Nasopharyngeal angiofibroma was first described by Hippocrates, a Greek physician, in the 5th century BC.
Classification
- Nasopharyngeal angiofibroma may be classified according to Radkowski Classification System into 3 categories:[1]
- Stage I
- Ia: limited to nasal cavity/nasopharynx
- Ib: extension into one or more paranasal sinuses
- Stage II
- IIa: minimal extension through sphenopalatine foramen into pterygomaxillary fossa
- IIb: fills pterygomaxillary fossa bowing the posterior wall of the maxillary antrum anteriorly or extending into the orbit via the inferior orbital fissure
- IIc: extends beyond pterygomaxillary fossa into infratemporal fossa
- Stage III
- Stage IIIA: intracranial extension
Pathophysiology
- The pathogenesis of nasopharyngeal angiofibroma is characterized by the following features:
- Vascular neoplasm
- Originates from the pterygopalatine fossa
- The majority are associated with the external carotid artery
- Genetic alterations associated with the development of nasopharyngeal angiofibroma include:[2]
- Overexpression PDGF-B
- Overexpression bFGF
- Overexpression bFGF
- Deletion of chromosome 17
- Tumor suppressor gene p53
- Overexpression of Her-2/neu oncogene
- On gross pathology, characteristic findings of nasopharyngeal angiofibroma include:
- Unencapsulated
- Polypoid fibrous mass
- Bleeding on manipulation
- On microscopic histopathological analysis, characteristic findings of nasopharyngeal angiofibroma include:
- Fibroblastic cells with plump (near cuboidal) nuclei
- Fibrous stroma
- Abundant capillaries
Causes
- There are no known causes of nasopharyngeal angiofibroma.
Differentiating Nasopharyngeal Angiofibroma from Other Diseases
- Nasopharyngeal angiofibroma must be differentiated from other diseases that cause epistaxis, unilateral nasal obstruction, and rhinorrhea, such as:
- Antro-choanal polyp
- Rhinosporidiosis
- Chordoma
- Nasopharanageal cyst
- Pyogenic granuloma
Epidemiology and Demographics
- Nasopharyngeal angiofibroma is a rare tumor.
- Nasopharyngeal angiofibroma accounts for 0.05% of all head and neck tumors.
- The prevalence of nasopharyngeal angiofibroma is approximately 0.4 per 100,000 individuals worldwide.[1]
Age
- Nasopharyngeal angiofibroma is more commonly observed among patients aged 7-19 years.[1]
Gender
- The male to female ratio for nasopharyngeal angiofibroma is approximately 4 to 1.[1]
Race
- There is no racial predilection for nasopharyngeal angiofibroma.
Risk Factors
- Common risk factors in the development of nasopharyngeal angiofibroma include:
- Presence of tumor in the pterygoid fossa
- Young age
- Feeders from the internal carotid artery
- Residual tumor
Natural History, Complications and Prognosis
- The majority of patients with nasopharyngeal angiofibroma are symptomatic at diagnosis.
- Early clinical features include epistaxis, facial pain, and headache.
- If left untreated, the majority of patients with nasopharyngeal angiofibroma may progress to develop malignant transformation.
- Common complications of nasopharyngeal angiofibroma include transient blindness, optic nerve damage, and low-grade consumption coagulopathy.
- Prognosis is generally good, and the 5-year survival rate of patients with early stage nasopharyngeal angiofibroma is approximately 90%
- Survival rate of patients with late stage nasopharyngeal angiofibroma is approximately 50% to 75%.
Diagnosis
Diagnostic Criteria
- The diagnosis of nasopharyngeal angiofibroma is made when the following diagnostic criteria are met:
- Clinical criteria
- Young patient
- Epistaxis
- Positive physical exam
- A smooth submucosal mass in the posterior nasal cavity
- Positive imaging finding: visualization of a nasopharyngeal mass
Symptoms
- Common symptoms of nasopharyngeal angiofibroma, may include:
- Epistaxis or blood-tinged nasal discharge
- Unilateral nasal obstruction
- Rhinorrhea
- Hearing loss
- Diplopia
- Anosmia (rare)
- Eye pain
Physical Examination
- Patients with nasopharyngeal angiofibroma usually are well-appearing.
- Physical examination may be remarkable for:
- A smooth submucosal mass in the posterior nasal cavity
Laboratory Findings
- There are no specific laboratory findings associated with the diagnosis of nasopharyngeal angiofibroma.
Imaging Findings
- Computed tomography is the imaging modality of choice for nasopharyngeal angiofibroma.
- On conventional radiography, findings of nasopharyngeal angiofibroma include:
- Visualization of a nasopharyngeal mass
- Opacification of the sphenoid sinus
- Anterior bowing of the posterior wall of the maxillary antrum
- Holman-miller sign: the anterior bowing of the posterior wall of the maxillary antrum which is seen on lateral skull film or cross-sectional imaging
- Widening of the pterygomaxillary fissure and pterygopalatine fossa
- Erosion of the medial pterygoid plate
- On CT, findings of nasopharyngeal angiofibroma include:
- Bony changes
- Non-encapsulated soft tissue mass
- Anterior bowing the posterior wall of the maxillary antrum
- On MRI, findings of nasopharyngeal angiofibroma include:
- T1: intermediate signal
- T2: heterogeneous signal - flow voids appear dark
- T1 C+ (Gd): shows prominent enhancement
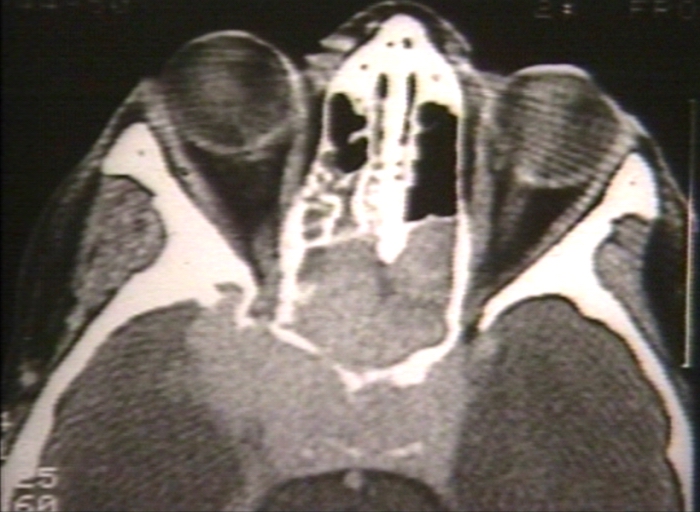
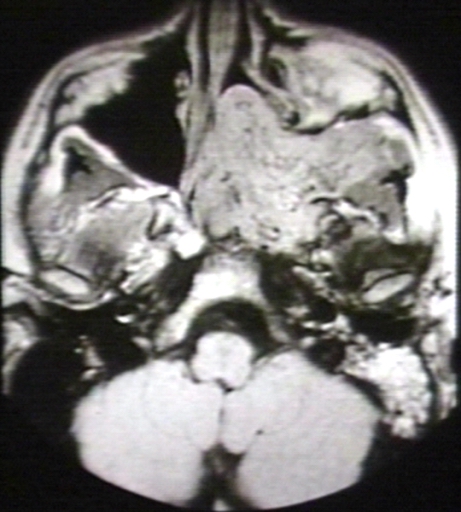
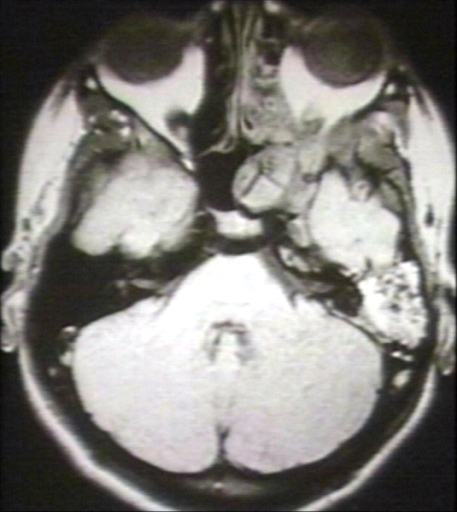
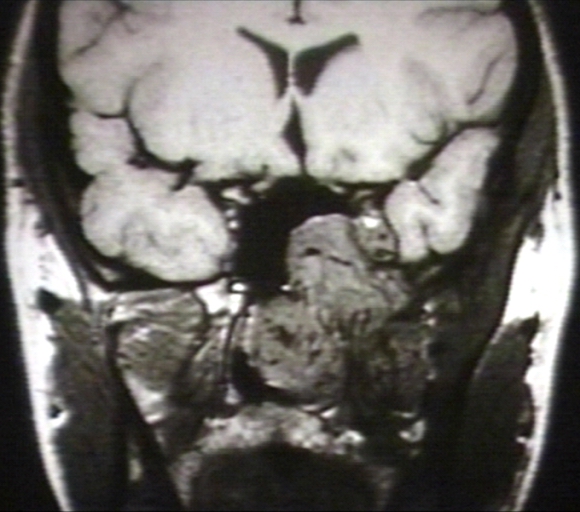
- The images below demonstrate findings of nasopharyngeal angiofibroma.
-
Nasopharyngeal angiofibroma. Skull base invasionImage courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology
-
MRI (T1): Nasopharyngeal angiofibroma 1/3 Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology
-
MRI (T1): Nasopharyngeal angiofibroma Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology
-
MRI (T1): Nasopharyngeal angiofibroma 3/3 Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology
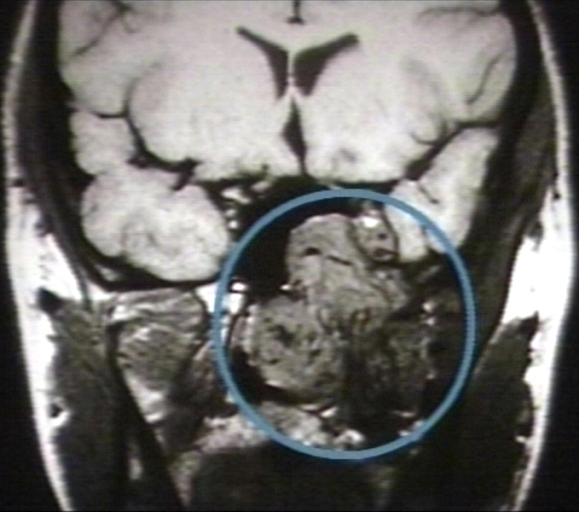
-
MRI (T1): Nasopharyngeal angiofibroma 3/3 (circled)Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology
Other Diagnostic Studies
- Nasopharyngeal angiofibroma may also be diagnosed using nasal endoscopy.
Treatment
Medical Therapy
- Medical therapy for nasopharyngeal angiofibroma is divided into 2 categories:[3]
- Hormonal therapy for nasopharyngeal angiofibroma includes:[3]
- Medical treatment is usually given before surgery to reduce the blood loss
- Radiotherapy for nasopharyngeal angiofibroma, include:
- Stereotactic radiotherapy
Surgery
- Surgery is the mainstay of therapy for nasopharyngeal angiofibroma.[3]
- Preoperative embolization is highly suggested for nasopharyngeal angiofibroma.
- Surgical approach for nasopharyngeal angiofibroma will depend on the stage.[3]
- The treatment of choice for early stage for nasopharyngeal angiofibroma is intranasal endoscopic surgery. Lateral rhinotomy is the preferred surgical approach.
- The treatment of choice for late stage for nasopharyngeal angiofibroma include the infratemporal fossa approach, and the mid-facial degloving approach.
Prevention
- There are no primary preventive measures available for nasopharyngeal angiofibroma.
- Once diagnosed and successfully treated, patients with nasopharyngeal angiofibroma are followed-up after surgery, and every 6 or 12 months, depending on the clinical progression.
References
- ↑ 1.0 1.1 1.2 1.3 Paris J, Guelfucci B, Moulin G, Zanaret M, Triglia JM (2001). "Diagnosis and treatment of juvenile nasopharyngeal angiofibroma". Eur Arch Otorhinolaryngol. 258 (3): 120–4. PMID 11374252.
- ↑ Coutinho-Camillo CM, Brentani MM, Nagai MA (2008). "Genetic alterations in juvenile nasopharyngeal angiofibromas". Head Neck. 30 (3): 390–400. doi:10.1002/hed.20775. PMID 18228521.
- ↑ 3.0 3.1 3.2 3.3 Nicolai P, Schreiber A, Bolzoni Villaret A (2012). "Juvenile angiofibroma: evolution of management". Int J Pediatr. 2012: 412545. doi:10.1155/2012/412545. PMC 3228400. PMID 22164185.