Mycophenolate sodium
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 94% (mofetil), 72% (sodium) |
| Protein binding | 97% |
| Metabolism | Hepatic |
| Elimination half-life | 16–18 hours |
| Excretion | Renal 93% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
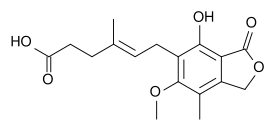
| Formula | C17H20O6 |
| Molar mass | 320.34 g.mol−1 |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Mycophenolic acid (INN) (IPA: Template:IPA) or mycophenolate is an immunosuppressant drug used to prevent rejection in organ transplantation. It was initially marketed as the prodrug mycophenolate mofetil (abbreviated MMF) to improve oral bioavailability. More recently, the salt mycophenolate sodium has also been introduced. Mycophenolic acid is commonly marketed under the trade names CellCept (mycophenolate mofetil; Roche) and Myfortic (mycophenolate sodium; Novartis).
Pharmacokinetics/pharmacology
Mycophenolate is derived from the fungus Penicillium stoloniferum. Mycophenolate mofetil is metabolised in the liver to the active moiety mycophenolic acid. It inhibits inosine monophosphate dehydrogenase, the enzyme which controls the rate of synthesis of guanine monophosphate in the de novo pathway of purine synthesis used in the proliferation of B and T lymphocytes.
Mycophenolate is potent and can be used in place of the older anti-proliferative azathioprine. It is usually used as part of triple-therapy including a calcineurin inhibitor (cyclosporin or tacrolimus) and prednisolone.
Clinical use
Indications
Generally speaking, mycophenolate is used for the prevention of organ transplant rejection. Specifically, mycophenolate mofetil is indicated for the prevention of organ transplant rejection in adults and renal transplant rejection in children >2 years; while mycophenolate sodium is indicated for the prevention of renal transplant rejection in adults. Mycophenolate sodium has also been used for the prevention of rejection in liver, heart and/or lung transplants in children >2 years.[1]
Recently, several studies have shown that oral mycophenolate mofetil is effective in inducing and maintaining remission in lupus nephritis.[2] It has been shown to be more effective and have fewer adverse effects than intravenous cyclophosphamide for lupus nephritis and has now become accepted first-line treatment for this disorder.
Adverse effects
Common adverse drug reactions (≥1% of patients) associated with mycophenolate therapy include: diarrhea, nausea, vomiting, infections, leukopenia, and/or anemia. Mycophenolate sodium is also commonly associated with fatigue, headache and/or cough. Intravenous (IV) administration of mycophenolate mofetil is also commonly associated with thrombophlebitis and thrombosis. Infrequent adverse effects (0.1–1% of patients) include: esophagitis, gastritis, gastrointestinal tract hemorrhage, and/or invasive cytomegalovirus (CMV) infection.[1]
Comparison to other agents
Compared with azathioprine it is more lymphocyte-specific and is associated with less bone marrow suppression, fewer opportunistic infections and lower incidence of acute rejection.[3] The exact role of mycophenolate vs azathioprine has yet to be conclusively established, but many centers use it in place of azathioprine for high-risk patients, or patients who have already experienced an episode of acute rejection. In long-term immunosuppression, it may be used to avoid calcineurin inhibitors or steroids.
Potential future uses
Mycophenolate mofetil is beginning to be used in the management of idiopathic thrombocytopenic purpura (ITP) and systemic lupus erythematosus (SLE) with success for some patients.
It is also currently being used as a long term therapy for maintaining remission of Wegener's Granulomatosis. It is being studied, along with ribavirin, for use against dengue.
Footnotes
- ↑ 1.0 1.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ↑ Ginzler EM, Dooley MA, Aranow C, Kim MY, et al. "Mycophenolate Mofetil or Intravenous Cyclophosphamide for Lupus Nephritis." New England Journal of Medicine. 353:21. 2219-2229. 24 November 2005.
- ↑ Woodroffe R, Yao G, Meads C, Bayliss S, Ready A, Raftery J, et al. Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study. Health Technol Assess 2005;9(21):1-194. PMID 15899149
External links
- MedlinePlus drug information: mycophenolate (systemic) – information from USP DI Advice for the Patient
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Immunosuppressive agents