Microsporidiosis pathophysiology: Difference between revisions
Ahmed Younes (talk | contribs) No edit summary |
Ahmed Younes (talk | contribs) No edit summary |
||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Microsporidia are a group of obligate intracellular parasitic fungi with more than 1,200 species belonging to 143 genera that infect a wide range of vertebrate and invertebrate hosts. They are characterized by the production of resistant spores that vary in size, depending on the species. | [[Microsporidia]] are a group of obligate [[Intracellular|intracellular parasitic]] [[fungi]] with more than 1,200 species belonging to 143 genera that infect a wide range of vertebrate and invertebrate hosts. They are characterized by the production of resistant spores that vary in size, depending on the species. | ||
==Pathophysiology== | ==Pathophysiology== | ||
| Line 11: | Line 11: | ||
{| style="float: right; width: 350px;" | {| style="float: right; width: 350px;" | ||
| [[Image:Sporoblast of the Microsporidium Fibrillanosema crangonycis. - By No machine-readable author provided. Javier martin assumed (based on copyright claims). - No machine-readable source provided. Own work assumed (based on copyright.png|right|400px|Life cycle of microsporoidosia - Public Domain, | | [[Image:Sporoblast of the Microsporidium Fibrillanosema crangonycis. - By No machine-readable author provided. Javier martin assumed (based on copyright claims). - No machine-readable source provided. Own work assumed (based on copyright.png|right|400px|Life cycle of microsporoidosia - Public Domain, </nowiki>https://commons.wikimedia.org/w/index.php?curid=2740681]] | ||
|} | |} | ||
#The spores enter the body via ingestion or inhalation. | #The spores enter the body via [[ingestion]] or [[inhalation]]. | ||
#Spores penetrate host cell using its polar tubule | #[[Spores]] penetrate host cell using its polar tubule. | ||
#The spore uses its polar tubule for injecting its sarcoplasm into the infected cell. | #The [[spore]] uses its polar tubule for injecting its [[sarcoplasm]] into the infected cell. | ||
#The sarcoplasm multiplies either by binary fission or multiple fission | #The [[sarcoplasm]] multiplies either by [[binary fission]] or multiple fission | ||
#The sarcoplasm of microsporidia develops into mature spores either free in the cytoplasm or inside a vacuole. | #The [[sarcoplasm]] of [[microsporidia]] develops into mature [[spores]] either free in the [[cytoplasm]] or inside a vacuole. | ||
#The process of development involves acquiring a thick capsule. This capsule provides protection against environmental stressors and is thought to play a role in its infectivity. | #The process of development involves acquiring a thick capsule. This [[capsule]] provides protection against environmental stressors and is thought to play a role in its infectivity. | ||
#After their number reach a certain limit, the host cell ruptures releasing the spores to continue the life cycle. | #After their number reach a certain limit, the host cell ruptures releasing the [[spores]] to continue the life cycle. | ||
===Pathogenesis=== | ===Pathogenesis=== | ||
*Microsporidia cause distortion of the villi without profound inflammatory findings. | *[[Microsporidia]] cause distortion of the [[villi]] without profound [[inflammatory]] findings. | ||
*With the progression of the disease, the microsporidia interfere with the intestinal absorption causing various symptoms of malabsorption (weight loss, chronic diarrhea, and vitamin deficiencies). | *With the progression of the disease, the [[microsporidia]] interfere with the intestinal absorption causing various symptoms of [[malabsorption]] ([[weight loss]], [[chronic diarrhea]], and [[vitamin deficiencies]]). | ||
*Disseminated disease can cause symptoms in the biliary tract, respiratory system, the urinary tract or the eye. | *Disseminated disease can cause symptoms in the [[biliary tract]], [[respiratory system]], the [[urinary tract]] or the eye. | ||
===Microscopic pathology=== | ===Microscopic pathology=== | ||
*Intestinal biopsy shows crypt hyperplasia and decrease in the absorptive surface of the intestine. | *Intestinal biopsy shows [[Crypt (anatomy)|crypt]] [[hyperplasia]] and decrease in the absorptive surface of the [[intestine]]. | ||
*Stool examination using “Quick-Hot Gram Chromotrope technique” reveals the spores staining dark violet and containing gram-positive granules. | *Stool examination using “Quick-Hot Gram Chromotrope technique” reveals the [[spores]] staining dark violet and containing [[gram-positive]] granules. | ||
*Enterocytozoon bieneusi spores measure 0.8-1.4 µm while spores of Anncaliia algerae, Encephalitozoon spp., Vittaforma corneae, and Nosema spp measure 1.5-4 µm. | *Enterocytozoon bieneusi spores measure 0.8-1.4 µm while spores of Anncaliia algerae, Encephalitozoon spp., Vittaforma corneae, and Nosema spp measure 1.5-4 µm. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 41: | Line 41: | ||
==Diseases caused by the different species== | ==Diseases caused by the different species== | ||
The clinical manifestations vary according to the causative species with diarrhea being the most common presentation.<ref name="urlCDC - DPDx - Microsporidiosis">{{cite web |url=https://www.cdc.gov/dpdx/microsporidiosis/index.html |title=CDC - DPDx - Microsporidiosis |format= |work= |accessdate=}}</ref> | The clinical manifestations vary according to the causative species with [[diarrhea]] being the most common presentation.<ref name="urlCDC - DPDx - Microsporidiosis">{{cite web |url=https://www.cdc.gov/dpdx/microsporidiosis/index.html |title=CDC - DPDx - Microsporidiosis |format= |work= |accessdate=}}</ref> | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 48: | Line 48: | ||
|- | |- | ||
|Anncaliia algerae | |Anncaliia algerae | ||
|Keratoconjunctivitis, skin and deep muscle infection | |[[Keratoconjunctivitis]], [[skin]] and deep muscle infection | ||
|- | |- | ||
|Enterocytozoon bieneusi* | |Enterocytozoon bieneusi* | ||
|Diarrhea, acalculous cholecystitis | |[[Diarrhea]], [[Cholecystitis|acalculous cholecystitis]] | ||
|- | |- | ||
|Encephalitozoon cuniculi and Encephalitozoon hellem | |Encephalitozoon cuniculi and Encephalitozoon hellem | ||
|Keratoconjunctivitis, infection of respiratory and genitourinary tract, disseminated infection | |[[Keratoconjunctivitis]], infection of [[respiratory]] and [[genitourinary tract]], disseminated infection | ||
|- | |- | ||
|Encephalitozoon intestinalis (syn. Septata intestinalis) | |Encephalitozoon intestinalis (syn. Septata intestinalis) | ||
|Infection of the GI tract causing diarrhea, and dissemination to ocular, genitourinary and respiratory tracts | |Infection of the GI tract causing [[diarrhea]], and dissemination to ocular, [[genitourinary]] and respiratory tracts | ||
|- | |- | ||
|Microsporidium (M. ceylonensis and M. africanum) | |Microsporidium (M. ceylonensis and M. africanum) | ||
|Infection of the cornea | |Infection of the [[cornea]] | ||
|- | |- | ||
|Nosema sp. (N. ocularum), Anncaliia connori | |Nosema sp. (N. ocularum), Anncaliia connori | ||
| Line 72: | Line 72: | ||
|- | |- | ||
|Trachipleistophora hominis | |Trachipleistophora hominis | ||
|Muscular infection, stromal keratitis, (probably disseminated infection) | |Muscular infection, [[Keratitis|stromal keratitis]], (probably disseminated infection) | ||
|- | |- | ||
|Tubulinosema acridophagus | |Tubulinosema acridophagus | ||
| Line 78: | Line 78: | ||
|- | |- | ||
|Vittaforma corneae (syn. Nosema corneum) | |Vittaforma corneae (syn. Nosema corneum) | ||
|Ocular infection, urinary tract infection | |Ocular infection, [[urinary tract infection]] | ||
|} | |} | ||
<nowiki>*</nowiki>Two reports of E. bieneusi in respiratory samples have also been published, one in 1992 and the other in 1997. | <nowiki>*</nowiki>Two reports of E. bieneusi in respiratory samples have also been published, one in 1992 and the other in 1997. | ||
Revision as of 14:12, 29 June 2017
|
Microsporidiosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Microsporidiosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Microsporidiosis pathophysiology |
|
Risk calculators and risk factors for Microsporidiosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Ogheneochuko Ajari, MB.BS, MS [2];Associate Editor(s)-in-Chief: Ahmed Younes M.B.B.CH [3]
Overview
Microsporidia are a group of obligate intracellular parasitic fungi with more than 1,200 species belonging to 143 genera that infect a wide range of vertebrate and invertebrate hosts. They are characterized by the production of resistant spores that vary in size, depending on the species.
Pathophysiology
Life Cycle
The infective form of microsporidia is the resistant spore and it can survive for an extended period of time in the environment.
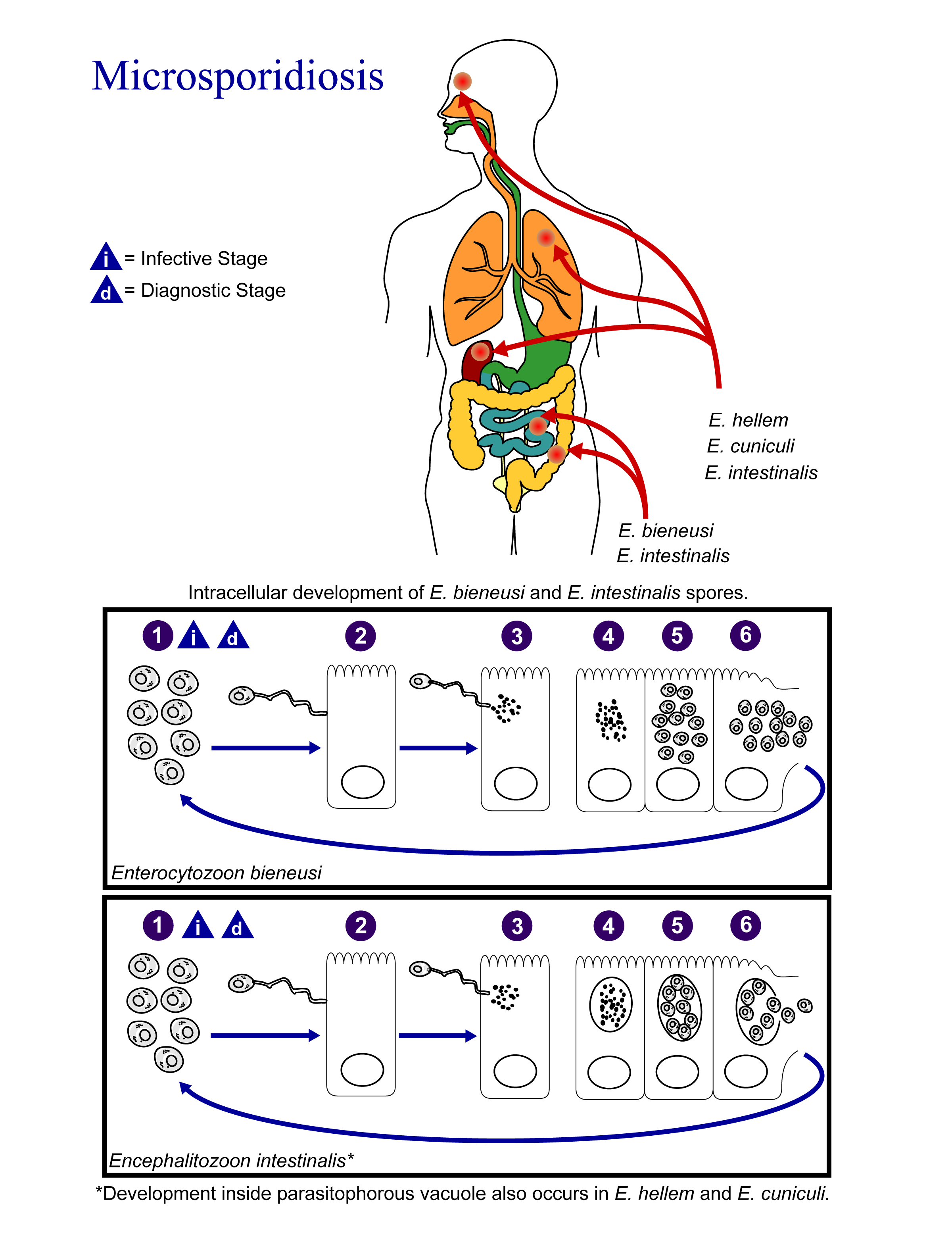 |
- The spores enter the body via ingestion or inhalation.
- Spores penetrate host cell using its polar tubule.
- The spore uses its polar tubule for injecting its sarcoplasm into the infected cell.
- The sarcoplasm multiplies either by binary fission or multiple fission
- The sarcoplasm of microsporidia develops into mature spores either free in the cytoplasm or inside a vacuole.
- The process of development involves acquiring a thick capsule. This capsule provides protection against environmental stressors and is thought to play a role in its infectivity.
- After their number reach a certain limit, the host cell ruptures releasing the spores to continue the life cycle.
Pathogenesis
- Microsporidia cause distortion of the villi without profound inflammatory findings.
- With the progression of the disease, the microsporidia interfere with the intestinal absorption causing various symptoms of malabsorption (weight loss, chronic diarrhea, and vitamin deficiencies).
- Disseminated disease can cause symptoms in the biliary tract, respiratory system, the urinary tract or the eye.
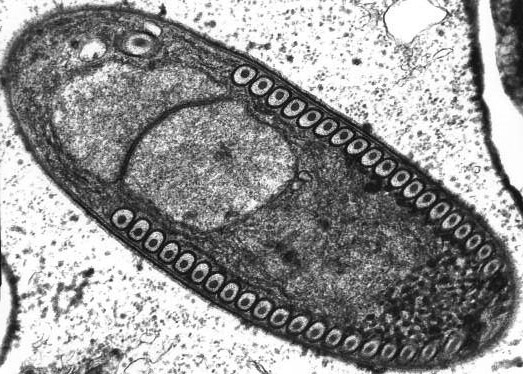
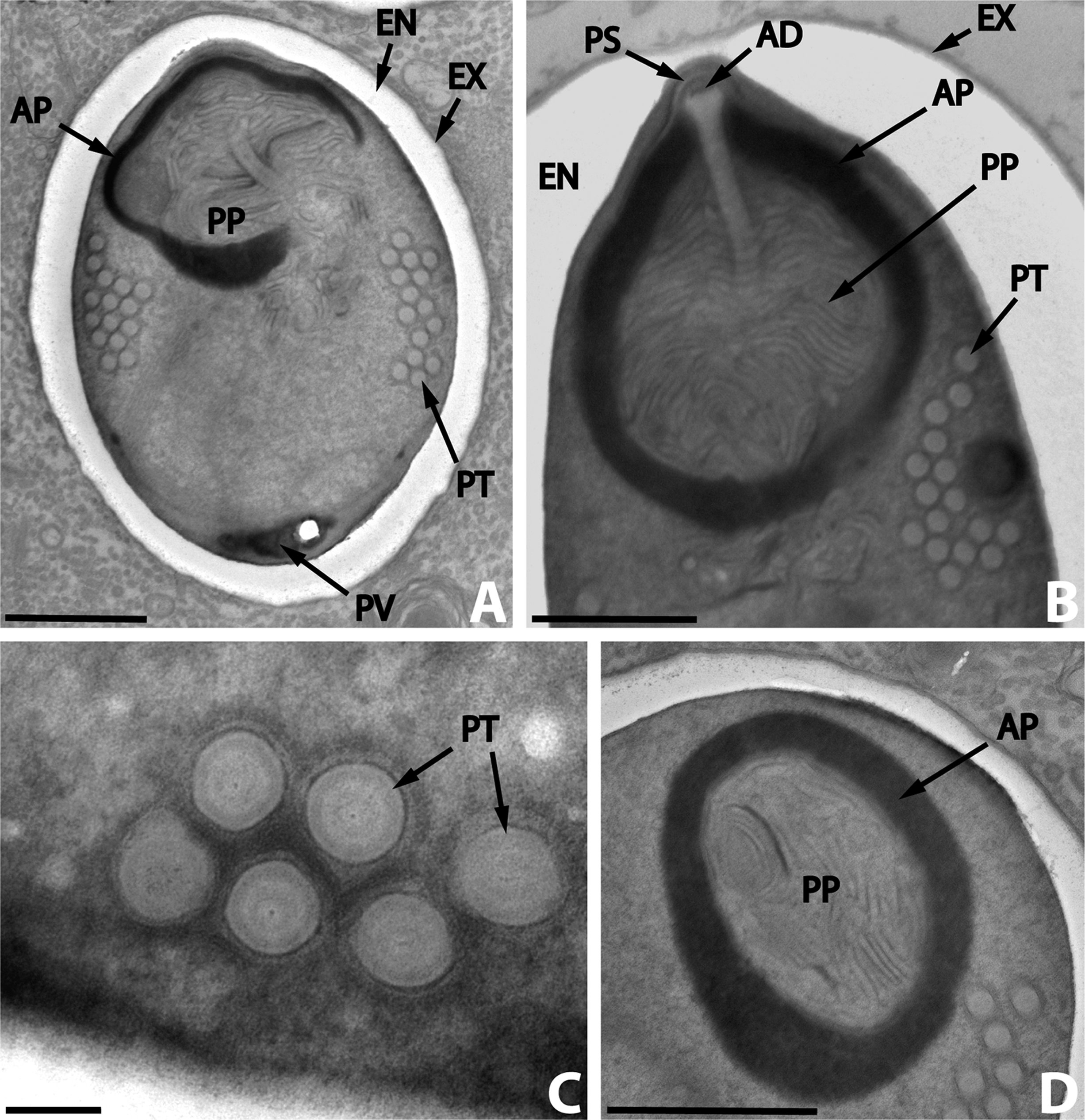
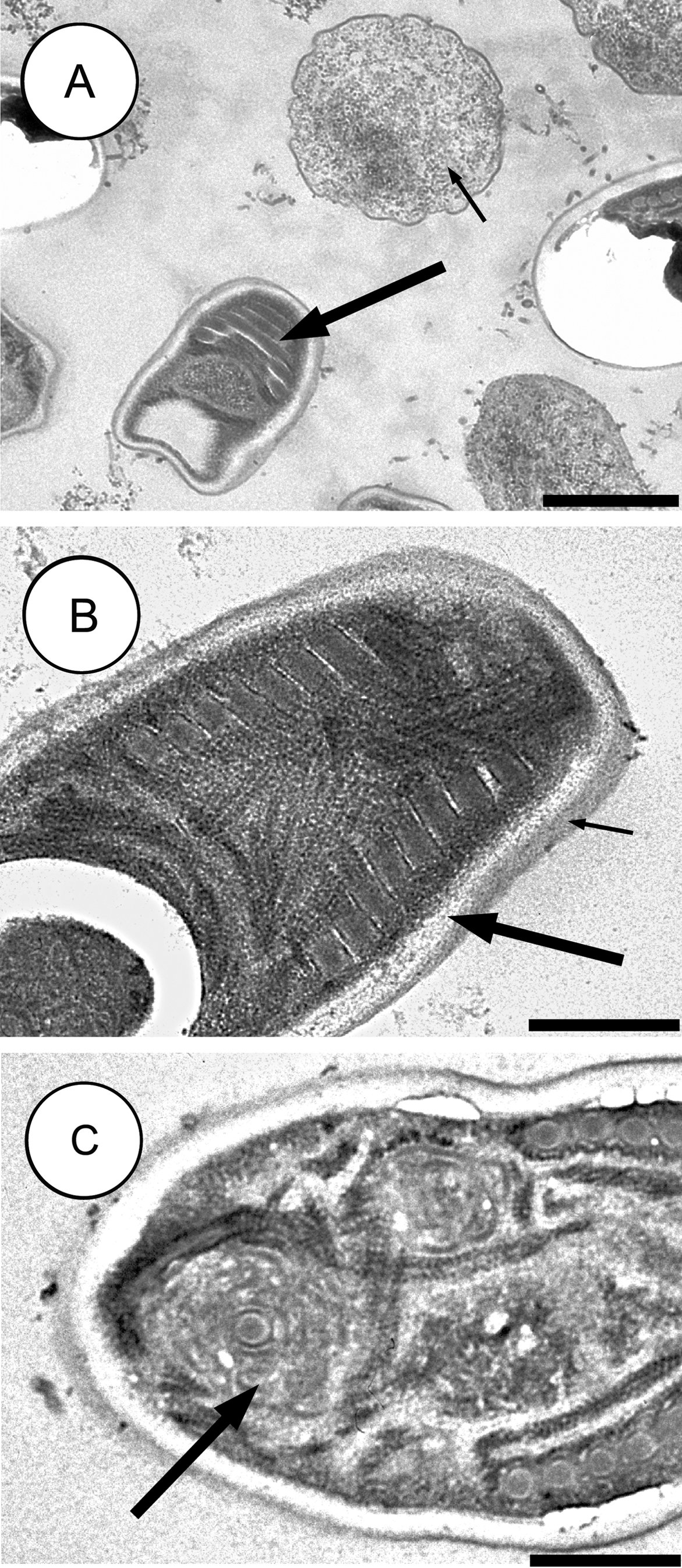
Microscopic pathology
- Intestinal biopsy shows crypt hyperplasia and decrease in the absorptive surface of the intestine.
- Stool examination using “Quick-Hot Gram Chromotrope technique” reveals the spores staining dark violet and containing gram-positive granules.
- Enterocytozoon bieneusi spores measure 0.8-1.4 µm while spores of Anncaliia algerae, Encephalitozoon spp., Vittaforma corneae, and Nosema spp measure 1.5-4 µm.
 |
 |
 |
|---|
Diseases caused by the different species
The clinical manifestations vary according to the causative species with diarrhea being the most common presentation.[1]
| Microsporidian species | Clinical manifestation |
| Anncaliia algerae | Keratoconjunctivitis, skin and deep muscle infection |
| Enterocytozoon bieneusi* | Diarrhea, acalculous cholecystitis |
| Encephalitozoon cuniculi and Encephalitozoon hellem | Keratoconjunctivitis, infection of respiratory and genitourinary tract, disseminated infection |
| Encephalitozoon intestinalis (syn. Septata intestinalis) | Infection of the GI tract causing diarrhea, and dissemination to ocular, genitourinary and respiratory tracts |
| Microsporidium (M. ceylonensis and M. africanum) | Infection of the cornea |
| Nosema sp. (N. ocularum), Anncaliia connori | Ocular infection |
| Pleistophora sp. | Muscular infection |
| Trachipleistophora anthropophthera | Disseminated infection |
| Trachipleistophora hominis | Muscular infection, stromal keratitis, (probably disseminated infection) |
| Tubulinosema acridophagus | Disseminated infection |
| Vittaforma corneae (syn. Nosema corneum) | Ocular infection, urinary tract infection |
*Two reports of E. bieneusi in respiratory samples have also been published, one in 1992 and the other in 1997.