Mesoridazine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 57: | Line 57: | ||
}} | }} | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} | ||
==Overview== | ==Overview== | ||
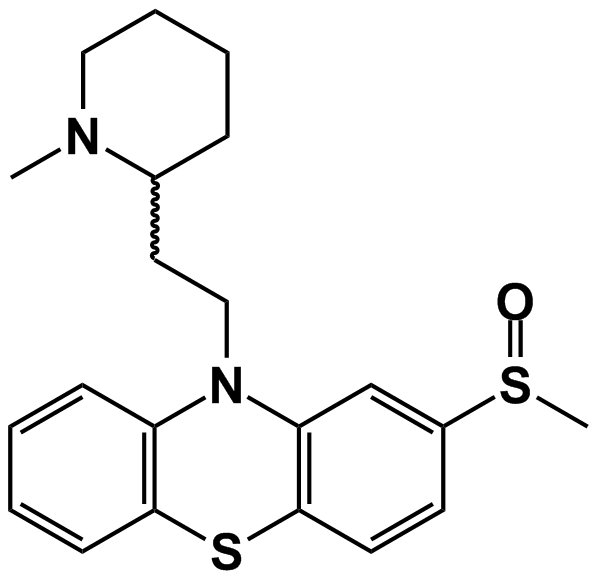
'''Mesoridazine''' ('''Serentil''') is a [[piperidine]] [[neuroleptic]] drug belonging to the class of drugs called [[phenothiazine]]s, used in the treatment of [[schizophrenia]]. It is a metabolite of [[thioridazine]]. The drug's name is derived from the '''me'''thyl'''s'''ulf'''o'''xy and pipe'''rid'''ine functional groups in its chemical structure. | '''Mesoridazine''' ('''Serentil''') is a [[piperidine]] [[neuroleptic]] drug belonging to the class of drugs called [[phenothiazine]]s, used in the treatment of [[schizophrenia]]. It is a metabolite of [[thioridazine]]. The drug's name is derived from the '''me'''thyl'''s'''ulf'''o'''xy and pipe'''rid'''ine functional groups in its chemical structure. | ||
| Line 76: | Line 73: | ||
Mesoridazine (10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylsufinyl)phenothiazine) is synthesized by an analogous scheme to that seen already for [[thioridazine]]. | Mesoridazine (10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylsufinyl)phenothiazine) is synthesized by an analogous scheme to that seen already for [[thioridazine]]. | ||
[[File:Mesoridazine synthesis.png| | [[File:Mesoridazine synthesis.png|400px|none|left]] | ||
However, it is also synthesized by alkylating the acidic form of 2-methylthiophenothiazine -methylsulfonylphenothiazine- using 2-(2-chloroethyl)-1-methylpiperidine. 2-methylthiophenothiazine is initially acylated at the nitrogen atom using [[acetic anhydride]], giving 10-acetyl-2-methylthiophenothiazine. The resulting acetyl derivative is further oxidized by [[hydrogen peroxide]] into 10-acetyl-2-methylsulfonylpenothiazine. Deacylation of this product in [[potassium carbonate]] methanol solution gives 2-methylsulfanylphenothiazine, which is alkylated by 2-(2-chlorethyl)-1-methylpiperidine in the presence of [[sodamide]], affording the desired mesoridazine. | However, it is also synthesized by alkylating the acidic form of 2-methylthiophenothiazine -methylsulfonylphenothiazine- using 2-(2-chloroethyl)-1-methylpiperidine. 2-methylthiophenothiazine is initially acylated at the nitrogen atom using [[acetic anhydride]], giving 10-acetyl-2-methylthiophenothiazine. The resulting acetyl derivative is further oxidized by [[hydrogen peroxide]] into 10-acetyl-2-methylsulfonylpenothiazine. Deacylation of this product in [[potassium carbonate]] methanol solution gives 2-methylsulfanylphenothiazine, which is alkylated by 2-(2-chlorethyl)-1-methylpiperidine in the presence of [[sodamide]], affording the desired mesoridazine. | ||
| Line 84: | Line 81: | ||
{{Antipsychotics}} | {{Antipsychotics}} | ||
[[Category:Phenothiazines]] | [[Category:Phenothiazines]] | ||
Latest revision as of 15:12, 13 April 2015
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Serentil |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682306 |
| Pregnancy category |
|
| Routes of administration | oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 4% |
| Metabolism | Hepatic/Renal |
| Elimination half-life | 24 to 48 hours |
| Excretion | Biliary and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H26N2OS2 |
| Molar mass | 386.576 g/mol |
| 3D model (JSmol) | |
| Melting point | 130 °C (266 °F) |
| Solubility in water | insoluble mg/mL (20 °C) |
| |
| |
| (verify) | |
|
WikiDoc Resources for Mesoridazine |
|
Articles |
|---|
|
Most recent articles on Mesoridazine Most cited articles on Mesoridazine |
|
Media |
|
Powerpoint slides on Mesoridazine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Mesoridazine at Clinical Trials.gov Clinical Trials on Mesoridazine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Mesoridazine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Mesoridazine Discussion groups on Mesoridazine Patient Handouts on Mesoridazine Directions to Hospitals Treating Mesoridazine Risk calculators and risk factors for Mesoridazine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Mesoridazine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Overview
Mesoridazine (Serentil) is a piperidine neuroleptic drug belonging to the class of drugs called phenothiazines, used in the treatment of schizophrenia. It is a metabolite of thioridazine. The drug's name is derived from the methylsulfoxy and piperidine functional groups in its chemical structure.
It has central antiadrenergic, antidopaminergic, antiserotonergic and weak muscarinic anticholinergic effects.
Serious side effects include akathisia, tardive dyskinesia and the potentially fatal neuroleptic malignant syndrome.
Mesoridazine was withdrawn from the United States market in 2004 due to dangerous side effects, namely irregular heart beat and QT-prolongation of the electrocardiogram.[1]
It currently appears to be unavailable worldwide.
Chemistry
Mesoridazine (10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylsufinyl)phenothiazine) is synthesized by an analogous scheme to that seen already for thioridazine.

However, it is also synthesized by alkylating the acidic form of 2-methylthiophenothiazine -methylsulfonylphenothiazine- using 2-(2-chloroethyl)-1-methylpiperidine. 2-methylthiophenothiazine is initially acylated at the nitrogen atom using acetic anhydride, giving 10-acetyl-2-methylthiophenothiazine. The resulting acetyl derivative is further oxidized by hydrogen peroxide into 10-acetyl-2-methylsulfonylpenothiazine. Deacylation of this product in potassium carbonate methanol solution gives 2-methylsulfanylphenothiazine, which is alkylated by 2-(2-chlorethyl)-1-methylpiperidine in the presence of sodamide, affording the desired mesoridazine.