Medrysone: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{KS}} | |||
|genericName=medrysone | |||
|aOrAn=a | |aOrAn=a | ||
|indicationType=treatment | |indicationType=treatment | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions=<!--Black Box Warning--> | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
| Line 12: | Line 13: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=== | |fdaLIADAdult===Indications== | ||
* | * HMS® (medrysone ophthalmic suspension) is indicated for the treatment of allergic [[conjunctivitis]], vernal [[conjunctivitis]], [[episcleritis]], and epinephrine sensitivity. | ||
==Dosage== | |||
= | * Shake well before using. Instill one drop into the conjunctival sac up to every four hours. | ||
|offLabelAdultGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=* There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
* | |offLabelPedNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
* | |contraindications=* HMS® suspension is contraindicated in most viral diseases of the cornea and conjunctiva, including epithelial [[herpes simplex keratitis]] (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. HMS® suspension is also contraindicated in individuals with known or suspected [[hypersensitivity]] to any of the ingredients of this preparation and to other corticosteroids. | ||
|warnings=* HMS® (medrysone ophthalmic suspension) is not recommended for use in [[iritis]] and uveitis as its therapeutic effectiveness has not been demonstrated in these conditions. | |||
* Prolonged use of corticosteroids may result in [[glaucoma]] with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Prolonged use may also suppress the host immune response and thus increase the hazard of secondary ocular infections. | |||
* Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation. | |||
* Acute purulent infections of the eye may be masked or activity enhanced by the presence of corticosteroid medication. | |||
* If this product is used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients. | |||
* | * Steroids should be used with caution in the presence of [[glaucoma]]. Intraocular pressure should be checked frequently. | ||
* The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. | |||
* Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution; frequent slit lamp microscopy is recommended. | |||
* Corticosteroids are not effective in mustard gas [[keratitis]] and Sjögren's [[keratoconjunctivitis]]. | |||
|clinicalTrials=* Adverse reactions include, in decreasing order of frequency, elevation of intraocular pressure (IOP) with possible development of glaucoma and infrequent optic nerve damage, posterior subcapsular cataract formation, and delayed wound healing. | |||
* Although systemic effects are extremely uncommon, there have been rare occurrences of systemic hypercorticoidism after use of topical steroids. | |||
* Corticosteroid-containing preparations have also been reported to cause acute anterior [[uveitis]] and perforation of the globe. [[Keratitis]], [[conjunctivitis]], [[corneal ulcers]], [[mydriasis]], conjunctival [[hyperemia]], loss of accommodation and [[ptosis]] have occasionally been reported following local use of corticosteroids. | |||
* The development of secondary ocular infection (bacterial, fungal and viral) has occurred. Fungal and viral infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids. The possibility of fungal invasion should be considered in any persistent corneal ulceration where steroid treatment has been used. | |||
* | * Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of HMS® suspension. Other adverse events reported with the use of HMS® suspension include: allergic reactions, foreign body sensation, and visual disturbance (blurry vision). | ||
|postmarketing=* There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
| | |||
|useInPregnancyFDA='''Teratogenic effects. Pregnancy Category C'''. Medrysone has been shown to be embryocidal in rabbits when given in doses 10 and 30 times the human ocular dose. Two drops of medrysone were applied to both eyes of pregnant rabbits 4 times per day on day 6 through 18 of gestation. A significant increase in early resorptions was observed in the treated rabbits. There are no adequate and well-controlled studies of medrysone in pregnant women. Medrysone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
|useInPregnancyFDA= | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Because of the potential for serious adverse reactions in nursing infants from medrysone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
|useInPed= | |useInPed=* Safety and effectiveness in pediatric patients below the age of 3 years have not been established. | ||
|useInGeri= | |useInGeri=* No overall differences in safety or effectiveness have been observed between elderly and younger patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
| Line 263: | Line 84: | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|administration=* Oral | |administration=* Oral | ||
| Line 275: | Line 96: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose==== | |overdose=* Overdosage will not ordinarily cause acute problems. If accidentally ingested, drink fluids to dilute. | ||
|drugBox={{drugbox2 | |||
| IUPAC_name = (6''S'',8''S'',9''S'',10''R'',11''S'',13''R'',14''S'',17''S'')- 17-acetyl-11-hydroxy-6,10,13-trimethyl-1,2,6,7,8,9,<br>11,12,14,15,16,17- dodecahydrocyclopenta[a] phenanthren-3-one | |||
| image =Medrysone.svg | |||
| CAS_number = 2668-66-8 | |||
| ATC_prefix = S01 | |||
| ATC_suffix = BA08 | |||
| ATC_supplemental = | |||
| PubChem = 247839 | |||
| DrugBank = APRD01091 | |||
| C=22 | H=32 | O=3 | |||
| molecular_weight = 344.488 g/mol | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = <!-- OTC / Rx-only --> | |||
| legal_status = | |||
| routes_of_administration = | |||
}} | |||
= | |mechAction=* | ||
<!--Structure--> | |||
|structure=* HMS® (medrysone ophthalmic suspension) 1% is a topical anti-inflammatory agent for ophthalmic use. | |||
* | |||
* '''Chemical Name''': | |||
:*11β-hydroxy-6α-methylpregn-4-ene-3,20-dione | |||
* Structural Formula: | |||
[[File:Medrysone structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | |||
* '''Contains''': Active: Medrysone 1%. Preservative: benzalkonium chloride 0.004%. | |||
: | * '''Inactives''': edetate disodium; hypromellose; polyvinyl alcohol 1.4%; potassium chloride; purified water; sodium chloride; sodium phosphate, dibasic; sodium phosphate, monobasic; and sodium hydroxide to adjust the pH (6.2 - 7.5). | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
| Line 307: | Line 145: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic= | |nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''': | ||
* No studies have been conducted in animals or in humans to evaluate the potential of these effects. | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | ||
|howSupplied=* HMS® (medrysone ophthalmic suspension) 1% is supplied sterile in opaque white LDPE plastic bottles with droppers with white high impact polystyrene (HIPS) caps as follows: | |||
[[File:Medrysone supply.png |thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|storage=* Store at temperatures up to 25°C (77°F). Protect from freezing. | |||
|packLabel=[[File:Medrysone.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo='''Information for Patients''': | |||
* If inflammation or pain persists longer than 48 hours or becomes aggravated, the patient should be advised to discontinue use of the medication and consult a physician. | |||
* This product is sterile when packaged. To prevent contamination, care should be taken to avoid touching the bottle tip to eyelids or to any other surface. The use of this bottle by more than one person may spread infection. Keep bottle tightly closed when not in use. Keep out of the reach of children. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* HMS®<ref>{{Cite web | title =medrysone suspension | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1612d931-d86e-4dba-bbd7-0aac3eaee131 }}</ref> | ||
|lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
Revision as of 13:49, 24 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Medrysone is a {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- HMS® (medrysone ophthalmic suspension) is indicated for the treatment of allergic conjunctivitis, vernal conjunctivitis, episcleritis, and epinephrine sensitivity.
Dosage
- Shake well before using. Instill one drop into the conjunctival sac up to every four hours.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Medrysone in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Medrysone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Medrysone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Medrysone in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Medrysone in pediatric patients.
Contraindications
- HMS® suspension is contraindicated in most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures. HMS® suspension is also contraindicated in individuals with known or suspected hypersensitivity to any of the ingredients of this preparation and to other corticosteroids.
Warnings
- HMS® (medrysone ophthalmic suspension) is not recommended for use in iritis and uveitis as its therapeutic effectiveness has not been demonstrated in these conditions.
- Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision, and in posterior subcapsular cataract formation. Prolonged use may also suppress the host immune response and thus increase the hazard of secondary ocular infections.
- Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal and scleral thinning. Use of topical corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation.
- Acute purulent infections of the eye may be masked or activity enhanced by the presence of corticosteroid medication.
- If this product is used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients.
- Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be checked frequently.
- The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
- Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution; frequent slit lamp microscopy is recommended.
- Corticosteroids are not effective in mustard gas keratitis and Sjögren's keratoconjunctivitis.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions include, in decreasing order of frequency, elevation of intraocular pressure (IOP) with possible development of glaucoma and infrequent optic nerve damage, posterior subcapsular cataract formation, and delayed wound healing.
- Although systemic effects are extremely uncommon, there have been rare occurrences of systemic hypercorticoidism after use of topical steroids.
- Corticosteroid-containing preparations have also been reported to cause acute anterior uveitis and perforation of the globe. Keratitis, conjunctivitis, corneal ulcers, mydriasis, conjunctival hyperemia, loss of accommodation and ptosis have occasionally been reported following local use of corticosteroids.
- The development of secondary ocular infection (bacterial, fungal and viral) has occurred. Fungal and viral infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids. The possibility of fungal invasion should be considered in any persistent corneal ulceration where steroid treatment has been used.
- Transient burning and stinging upon instillation and other minor symptoms of ocular irritation have been reported with the use of HMS® suspension. Other adverse events reported with the use of HMS® suspension include: allergic reactions, foreign body sensation, and visual disturbance (blurry vision).
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Medrysone in the drug label.
Drug Interactions
There is limited information regarding Medrysone Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Teratogenic effects. Pregnancy Category C. Medrysone has been shown to be embryocidal in rabbits when given in doses 10 and 30 times the human ocular dose. Two drops of medrysone were applied to both eyes of pregnant rabbits 4 times per day on day 6 through 18 of gestation. A significant increase in early resorptions was observed in the treated rabbits. There are no adequate and well-controlled studies of medrysone in pregnant women. Medrysone should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Medrysone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Medrysone during labor and delivery.
Nursing Mothers
- It is not known whether topical ophthalmic administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human breast milk. Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Because of the potential for serious adverse reactions in nursing infants from medrysone, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 3 years have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Medrysone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Medrysone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Medrysone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Medrysone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Medrysone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Medrysone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Medrysone in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Medrysone in the drug label.
Overdosage
- Overdosage will not ordinarily cause acute problems. If accidentally ingested, drink fluids to dilute.
Pharmacology
| Template:Px | |
Medrysone
| |
| Systematic (IUPAC) name | |
| (6S,8S,9S,10R,11S,13R,14S,17S)- 17-acetyl-11-hydroxy-6,10,13-trimethyl-1,2,6,7,8,9, 11,12,14,15,16,17- dodecahydrocyclopenta[a] phenanthren-3-one | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 344.488 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
Structure
- HMS® (medrysone ophthalmic suspension) 1% is a topical anti-inflammatory agent for ophthalmic use.
- Chemical Name:
- 11β-hydroxy-6α-methylpregn-4-ene-3,20-dione
- Structural Formula:
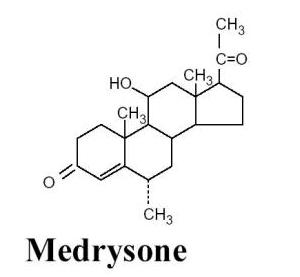
- Contains: Active: Medrysone 1%. Preservative: benzalkonium chloride 0.004%.
- Inactives: edetate disodium; hypromellose; polyvinyl alcohol 1.4%; potassium chloride; purified water; sodium chloride; sodium phosphate, dibasic; sodium phosphate, monobasic; and sodium hydroxide to adjust the pH (6.2 - 7.5).
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Medrysone in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Medrysone in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility:
- No studies have been conducted in animals or in humans to evaluate the potential of these effects.
Clinical Studies
There is limited information regarding Clinical Studies of Medrysone in the drug label.
How Supplied
- HMS® (medrysone ophthalmic suspension) 1% is supplied sterile in opaque white LDPE plastic bottles with droppers with white high impact polystyrene (HIPS) caps as follows:

Storage
- Store at temperatures up to 25°C (77°F). Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Medrysone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Medrysone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients:
- If inflammation or pain persists longer than 48 hours or becomes aggravated, the patient should be advised to discontinue use of the medication and consult a physician.
- This product is sterile when packaged. To prevent contamination, care should be taken to avoid touching the bottle tip to eyelids or to any other surface. The use of this bottle by more than one person may spread infection. Keep bottle tightly closed when not in use. Keep out of the reach of children.
Precautions with Alcohol
- Alcohol-Medrysone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- HMS®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "medrysone suspension".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Medrysone
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Medrysone |Label Name=Medrysone11.png
}}
{{#subobject:
|Label Page=Medrysone |Label Name=Medrysone11.png
}}