Liposarcoma pathophysiology: Difference between revisions
No edit summary |
|||
| Line 15: | Line 15: | ||
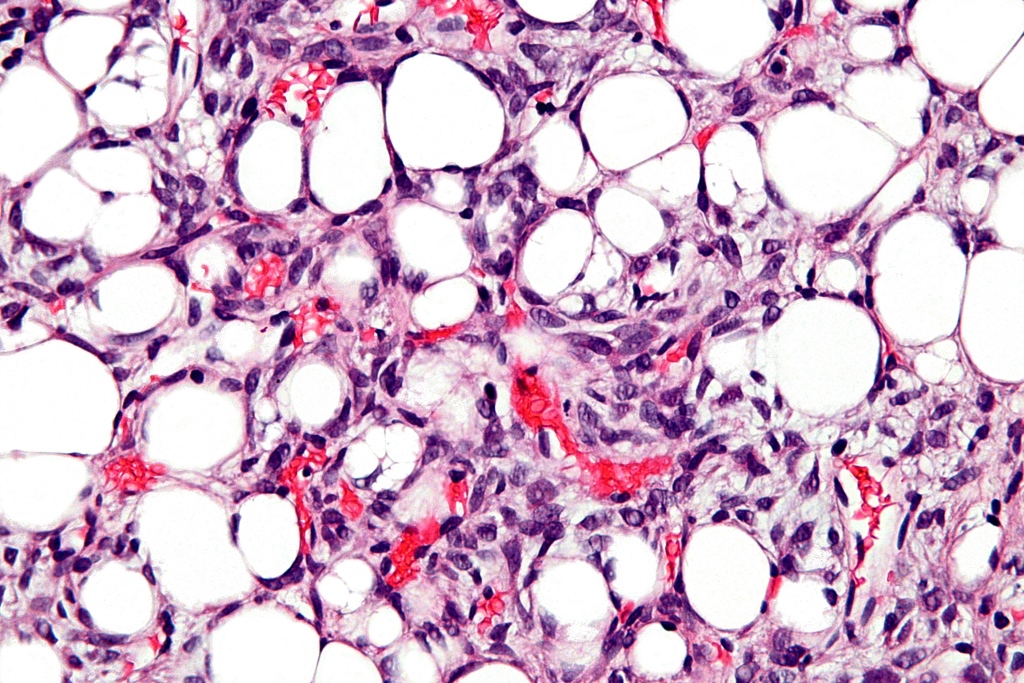
Frequently composed by [[adipocytes]] with different cell sizes, hyperchromasia, and nuclear atypia. [[Fibrous]] septa may be identified surrounding [[adipocytes]], containing hyperchromatic [[stromal cells]]. Besides these two types of cells, mono- or multi-vacuolated lipoblasts may also be identified. Lipoblasts are characterized by the presence of single (mono) or multiple (multi) peripheral cytoplasmic vacuoles that press on the hyperchromatic nucleus.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> In general, adipocytic [[neoplasms]] are often identified by the presence of these lipoblasts. However, the presence of lipoblasts is not specific, since multiple benign lesions may contain lipoblasts. Additionally, lipoblasts may infrequently be absent, which makes the diagnosis of adipocytic neoplasm more difficult but possible with the help of other histological features.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> | Frequently composed by [[adipocytes]] with different cell sizes, hyperchromasia, and nuclear atypia. [[Fibrous]] septa may be identified surrounding [[adipocytes]], containing hyperchromatic [[stromal cells]]. Besides these two types of cells, mono- or multi-vacuolated lipoblasts may also be identified. Lipoblasts are characterized by the presence of single (mono) or multiple (multi) peripheral cytoplasmic vacuoles that press on the hyperchromatic nucleus.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> In general, adipocytic [[neoplasms]] are often identified by the presence of these lipoblasts. However, the presence of lipoblasts is not specific, since multiple benign lesions may contain lipoblasts. Additionally, lipoblasts may infrequently be absent, which makes the diagnosis of adipocytic neoplasm more difficult but possible with the help of other histological features.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref> | ||
====Inflammatory Liposarcoma==== | ====Inflammatory Liposarcoma==== | ||
Its adipocitic nature may be misidentified due to the heavy chronic inflammatory infiltrate. The inflammatory component is frequently composed of different lympho-plasmacytic aggregates. These tend to be predominantly formed by a specific type of [[B-cell]], or less commonly [[T-cells]] may populate the inflammatory aggregate.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref><ref name="pmid9158675">{{cite journal| author=Kraus MD, Guillou L, Fletcher CD| title=Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma. | journal=Am J Surg Pathol | year= 1997 | volume= 21 | issue= 5 | pages= 518-27 | pmid=9158675 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9158675 }} </ref><ref name="pmid9255251">{{cite journal| author=Argani P, Facchetti F, Inghirami G, Rosai J| title=Lymphocyte-rich well-differentiated liposarcoma: report of nine cases. | journal=Am J Surg Pathol | year= 1997 | volume= 21 | issue= 8 | pages= 884-95 | pmid=9255251 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9255251 }} </ref> | Its adipocitic nature may be misidentified due to the heavy chronic inflammatory infiltrate. The inflammatory component is frequently composed of different lympho-plasmacytic aggregates. These tend to be predominantly formed by a specific type of [[B-cell]], or less commonly [[T-cells]] may populate the inflammatory aggregate.<ref name="pmid10982304">{{cite journal| author=Dei Tos AP| title=Liposarcoma: new entities and evolving concepts. | journal=Ann Diagn Pathol | year= 2000 | volume= 4 | issue= 4 | pages= 252-66 | pmid=10982304 | doi=10.1053/adpa.2000.8133 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10982304 }} </ref><ref name="pmid9158675">{{cite journal| author=Kraus MD, Guillou L, Fletcher CD| title=Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma. | journal=Am J Surg Pathol | year= 1997 | volume= 21 | issue= 5 | pages= 518-27 | pmid=9158675 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9158675 }} </ref><ref name="pmid9255251">{{cite journal| author=Argani P, Facchetti F, Inghirami G, Rosai J| title=Lymphocyte-rich well-differentiated liposarcoma: report of nine cases. | journal=Am J Surg Pathol | year= 1997 | volume= 21 | issue= 8 | pages= 884-95 | pmid=9255251 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9255251 }} </ref> | ||
'''Spindle cell lipocarcinoma''' is a rare adult-type of well-differentiated liposarcoma. It results from the proliferation of neural-like [[spindle cells]], which are organized in a fibrous structure, that contains [[lipoblasts]].<ref name="pmid8067512">{{cite journal| author=Dei Tos AP, Mentzel T, Newman PL, Fletcher CD| title=Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases. | journal=Am J Surg Pathol | year= 1994 | volume= 18 | issue= 9 | pages= 913-21 | pmid=8067512 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8067512 }} </ref><ref name="pmid5">{{cite journal| author=Hendrickson WA, Ward KB| title=Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin. | journal=Biochem Biophys Res Commun | year= 1975 | volume= 66 | issue= 4 | pages= 1349-56 | pmid=5 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5 }} </ref> | '''Spindle cell lipocarcinoma''' is a rare adult-type of well-differentiated liposarcoma. It results from the proliferation of neural-like [[spindle cells]], which are organized in a fibrous structure, that contains [[lipoblasts]].<ref name="pmid8067512">{{cite journal| author=Dei Tos AP, Mentzel T, Newman PL, Fletcher CD| title=Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases. | journal=Am J Surg Pathol | year= 1994 | volume= 18 | issue= 9 | pages= 913-21 | pmid=8067512 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8067512 }} </ref><ref name="pmid5">{{cite journal| author=Hendrickson WA, Ward KB| title=Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin. | journal=Biochem Biophys Res Commun | year= 1975 | volume= 66 | issue= 4 | pages= 1349-56 | pmid=5 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=5 }} </ref> | ||
Revision as of 17:16, 24 August 2015
|
Liposarcoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Liposarcoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Liposarcoma pathophysiology |
|
Risk calculators and risk factors for Liposarcoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Overview
The pathogenesis of liposarcoma highly depends on its subtype. The role of proto-oncogenes has been implicated in the disease development.
Pathogenesis
Each liposarcoma subtype has specific mechanisms of pathogenesis and characteristics:
Well-Differentiated Liposarcoma
This type of liposarcoma develops at the limbs and retroperitoneum in equal frequencies. Less commonly, it develops at the mediastinum and spermatic cord, which represent about 45% of liposarcomas.[1]
Sclerosing Liposarcoma
Occurs most frequently at the retroperitoneum and paratesticular regions. The particular histological finding in this type of well differentiated liposarcoma is the identification of distinctive stromal cells distributed across the tissue, which are associated with lipoblasts filled with multiple vacuoles. This association forms a collagenous background of fibrillary appearance. In certain cases, the fibrous component of the neoplasm may occupy most of its mass.[1]
Adipocytic Liposarcoma
Frequently composed by adipocytes with different cell sizes, hyperchromasia, and nuclear atypia. Fibrous septa may be identified surrounding adipocytes, containing hyperchromatic stromal cells. Besides these two types of cells, mono- or multi-vacuolated lipoblasts may also be identified. Lipoblasts are characterized by the presence of single (mono) or multiple (multi) peripheral cytoplasmic vacuoles that press on the hyperchromatic nucleus.[1] In general, adipocytic neoplasms are often identified by the presence of these lipoblasts. However, the presence of lipoblasts is not specific, since multiple benign lesions may contain lipoblasts. Additionally, lipoblasts may infrequently be absent, which makes the diagnosis of adipocytic neoplasm more difficult but possible with the help of other histological features.[1]
Inflammatory Liposarcoma
Its adipocitic nature may be misidentified due to the heavy chronic inflammatory infiltrate. The inflammatory component is frequently composed of different lympho-plasmacytic aggregates. These tend to be predominantly formed by a specific type of B-cell, or less commonly T-cells may populate the inflammatory aggregate.[1][2][3] Spindle cell lipocarcinoma is a rare adult-type of well-differentiated liposarcoma. It results from the proliferation of neural-like spindle cells, which are organized in a fibrous structure, that contains lipoblasts.[4][5]
Because well-differentiated lipomas are characterized by having approximately 30% risk of recurrence and no potential for metastasize unless dedifferentiation occurs, two terms have emerged: atypical lipoma and atypical lipomatous tumor. However, there is still no consensus on the appropriate use of these terms.[6]
Dedifferentiated Liposarcoma
In this form of liposarcoma there is a transition from a low-grade differentiation to a high-grade differentiation within the same mass of well-differentiated liposarcoma.[1][7][8] 90% od de-differentiation from well-differented liposarcomas occur in primary tumors, while the remaining 10% occur in reccurent neoplasms.
Myxoid Liposarcoma
They have a non-homogenous appearance with cystic and solid components. Ultrasound and MRI may be helpful to identify the coresponding cystic and solid structures.
Round Cell Liposarcoma
It is a high-grade liposarcoma which is a poorly differentiated form of myxoid sarcoma. It has a very poor prognosis and often metastasize to the retroperitoneum, pleural cavity, soft tissue, or pelvis and very rarely to the lungs. Microscopically it consists of small, round, or spindled cells with sparse eosinophilic and granular cytoplasm and large nuclei. It may have scattered lipoblasts and areas of necrosis.
Pleomorphic Liposarcoma
Pleomorphic liposarcoma is one of the rarest liposarcoma subtypes. Approximately 5% of all liposarcomas are pleomorphic liposarcomas. It may develop in the extremities and rarely retroperitoneally. When in the retroperitoneum, they are mostly metastatic.
Genetics
Well-Differentiated Liposarcoma
Specific diagnostic techniques, such as karyotyping, showed a specific characteristic of these cells, namely the presence of a large marker chromosome and/or of an extra ring.[9] With the help of fluorescence in-situ hybridization (FISH) technique, these rings and/or large markers were noted to accommodate amplified genetic sequences of the 12q13-15 chromosome region.[10] This chromosome region is rich in protooncogenes, including the CHOP, CDK4, MDM2, HMGI-C, GLI, SAS, OS1, and the OS9, all of which play an important role in the pathogenesis of many neoplasms. Amplification of some of these regions, with concomitant amplification of their proteins, was clearly demonstrated in liposarcoma.[11] Because this same region is rearranged in benign lipomas as well, benign lesions may develop to become well-defferentiated liposarcoma masses. The difference between malignant and benign masses resides in the amount of rearranged gene present in each tissue.[11]
Associated Conditions
Liposarcoma is associated with genetic conditions like Li- Fraumeni syndrome.
Gross Pathology
- Liposarcoma usually presents as a mass which often resembles lipoma and is multinodular.
- Cut surface may be soft or firm and is yellow in color.
- Focal mucinous area may be seen.
- Areas of necrosis may be noted.
Microscopic Pathology
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Dei Tos AP (2000). "Liposarcoma: new entities and evolving concepts". Ann Diagn Pathol. 4 (4): 252–66. doi:10.1053/adpa.2000.8133. PMID 10982304.
- ↑ Kraus MD, Guillou L, Fletcher CD (1997). "Well-differentiated inflammatory liposarcoma: an uncommon and easily overlooked variant of a common sarcoma". Am J Surg Pathol. 21 (5): 518–27. PMID 9158675.
- ↑ Argani P, Facchetti F, Inghirami G, Rosai J (1997). "Lymphocyte-rich well-differentiated liposarcoma: report of nine cases". Am J Surg Pathol. 21 (8): 884–95. PMID 9255251.
- ↑ Dei Tos AP, Mentzel T, Newman PL, Fletcher CD (1994). "Spindle cell liposarcoma, a hitherto unrecognized variant of liposarcoma. Analysis of six cases". Am J Surg Pathol. 18 (9): 913–21. PMID 8067512.
- ↑ Hendrickson WA, Ward KB (1975). "Atomic models for the polypeptide backbones of myohemerythrin and hemerythrin". Biochem Biophys Res Commun. 66 (4): 1349–56. PMID 5.
- ↑ Evans HL, Soule EH, Winkelmann RK (1979). "Atypical lipoma, atypical intramuscular lipoma, and well differentiated retroperitoneal liposarcoma: a reappraisal of 30 cases formerly classified as well differentiated liposarcoma". Cancer. 43 (2): 574–84. PMID 421182.
- ↑ Evans HL (1979). "Liposarcoma: a study of 55 cases with a reassessment of its classification". Am J Surg Pathol. 3 (6): 507–23. PMID 534388.
- ↑ Dahlin DC, Unni KK, Matsuno T (1977). "Malignant (fibrous) histiocytoma of bone--fact or fancy?". Cancer. 39 (4): 1508–16. PMID 192432.
- ↑ Rosai J, Akerman M, Dal Cin P, DeWever I, Fletcher CD, Mandahl N; et al. (1996). "Combined morphologic and karyotypic study of 59 atypical lipomatous tumors. Evaluation of their relationship and differential diagnosis with other adipose tissue tumors (a report of the CHAMP Study Group)". Am J Surg Pathol. 20 (10): 1182–9. PMID 8827023.
- ↑ Dal Cin P, Kools P, Sciot R, De Wever I, Van Damme B, Van de Ven W; et al. (1993). "Cytogenetic and fluorescence in situ hybridization investigation of ring chromosomes characterizing a specific pathologic subgroup of adipose tissue tumors". Cancer Genet Cytogenet. 68 (2): 85–90. PMID 8353809.
- ↑ 11.0 11.1 Dei Tos AP, Doglioni C, Piccinin S, Sciot R, Furlanetto A, Boiocchi M; et al. (2000). "Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours". J Pathol. 190 (5): 531–6. doi:10.1002/(SICI)1096-9896(200004)190:5<531::AID-PATH579>3.0.CO;2-W. PMID 10727978.