Levonorgestrel (oral): Difference between revisions
No edit summary |
No edit summary |
||
| Line 48: | Line 48: | ||
The most common adverse events (>10%) in the clinical trial for women receiving levonorgestrel tablet included heavier [[menstrual bleeding]] (30.9%), [[nausea]] (13.7%), [[lower abdominal pain]] (13.3%), [[fatigue]] (13.3%), and [[headache]] (10.3%). Table 1 lists those adverse events that were reported in > 4% of levonorgestrel tablet users. | The most common adverse events (>10%) in the clinical trial for women receiving levonorgestrel tablet included heavier [[menstrual bleeding]] (30.9%), [[nausea]] (13.7%), [[lower abdominal pain]] (13.3%), [[fatigue]] (13.3%), and [[headache]] (10.3%). Table 1 lists those adverse events that were reported in > 4% of levonorgestrel tablet users. | ||
[[file:Levonorgestrel Side Effects.png|none| | [[file:Levonorgestrel Side Effects.png|none|350px]] | ||
|postmarketing=The following adverse reactions have been identified during post-approval use of levonorgestrel tablets (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |postmarketing=The following adverse reactions have been identified during post-approval use of levonorgestrel tablets (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
| Line 68: | Line 68: | ||
*[[Oligomenorrhea]] | *[[Oligomenorrhea]] | ||
*[[Pelvic Pain]] | *[[Pelvic Pain]] | ||
|drugInteractions=Drugs or herbal products that induce enzymes, including [[CYP3A4]], that metabolize progestins may decrease the plasma concentrations of progestins, and may decrease the effectiveness of progestin-only pills. Some drugs or herbal products that may decrease the effectiveness of progestin-only pills include: | |||
*[[Barbiturates]] | |||
*[[Bosentan]] | |||
*[[Carbamazepine]] | |||
*[[Felbamate]] | |||
*[[Griseofulvin]] | |||
*[[Oxcarbazepine]] | |||
*[[Phenytoin]] | |||
*[[Rifampin]] | |||
*[[St. John’s wort]] | |||
*[[Topiramate]] | |||
Significant changes (increase or decrease) in the plasma levels of the progestin have been noted in some cases of coadministration with HIV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors. | |||
Consult the labeling of all concurrently used drugs to obtain further information about interactions with progestin-only pills or the potential for enzyme alterations. | |||
|useInPregnancyFDA=Many studies have found no harmful effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted with progestin-only pills have not demonstrated significant adverse effects. | |||
|alcohol=Alcohol-Levonorgestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Levonorgestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 15:12, 12 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Levonorgestrel (oral) is an emergency contraceptive that is FDA approved for the prophylaxis of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. To obtain optimal efficacy, the tablet should be taken as soon as possible within 72 hours of intercourse.. Common adverse reactions include menstrual bleeding, nausea, lower abdominal pain, fatigue, headache, and dizziness..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Emergency Contraceptive
- Dosage: Taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if the tablet is taken as soon as possible after unprotected intercourse. Each round tablet containing 1.5 mg of levonorgestrel.
Menorrhagia
- Dosage: Disposed as an IUD which releases 20 micrograms/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levonorgestrel in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levonorgestrel in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Levonorgestrel (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levonorgestrel in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levonorgestrel in pediatric patients.
Contraindications
Contraindicated for use in the case of known or suspected pregnancy.
Warnings
Ectopic Pregnancy
Ectopic pregnancies account for approximately 2% of all reported pregnancies. Up to 10% of pregnancies reported in clinical studies of routine use of progestin-only contraceptives are ectopic.
A history of ectopic pregnancy is not a contraindication to use of this emergency contraceptive method. Healthcare providers, however, should consider the possibility of an ectopic pregnancy in women who become pregnant or complain of lower abdominal pain after taking Levonorgestrel. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking Next Choice One DoseTM.
Existing Pregnancy
Levonorgestrel is not effective in terminating an existing pregnancy.
Effect on Menses
Some women may experience spotting a few days after taking Levonorgestrel. Menstrual bleeding patterns are often irregular among women using progestin-only oral contraceptives and women using levonorgestrel for postcoital and emergency contraception.
If there is a delay in the onset of expected menses beyond 1 week, consider the possibility of pregnancy.
STI/HIV
Levonorgestrel does not protect against HIV infection (AIDS) or other sexually transmitted infections (STIs).
Physical Examination and Follow-up
A physical examination is not required prior to prescribing Next Choice One DoseTM. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking Levonorgestrel.
Fertility Following Discontinuation
A rapid return of fertility is likely following treatment with Levonorgestrel for emergency contraception; therefore, routine contraception should be continued or initiated as soon as possible following use of Next Choice One DoseTM to ensure ongoing prevention of pregnancy.
Presence of FD&C Yellow
Levonorgestrel contains FD&C Yellow #6 as a color additive.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Levonorgestrel tablet was studied in a randomized, double-blinded multicenter clinical trial. In this study, all women who had received at least one dose of study medication were included in the safety analysis: 1,379 women in the levonorgestrel tablet group (1 dose of 1.5 mg levonorgestrel), and 1,377 women in the levonorgestrel tablets group (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). The mean age of women given levonorgestrel tablet was 27 years. The racial demographic of those enrolled was 54% Chinese, 12% Other Asian or Black, and 34% were Caucasian in each treatment group. 1.6% of women in the levonorgestrel tablet group and 1.4% in levonorgestrel tablets group were lost to follow-up.
The most common adverse events (>10%) in the clinical trial for women receiving levonorgestrel tablet included heavier menstrual bleeding (30.9%), nausea (13.7%), lower abdominal pain (13.3%), fatigue (13.3%), and headache (10.3%). Table 1 lists those adverse events that were reported in > 4% of levonorgestrel tablet users.

Postmarketing Experience
The following adverse reactions have been identified during post-approval use of levonorgestrel tablets (2 doses of 0.75 mg levonorgestrel taken 12 hours apart). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders
General Disorders and Administration Site Conditions
Nervous System Disorders
Reproductive System and Breast Disorders
Drug Interactions
Drugs or herbal products that induce enzymes, including CYP3A4, that metabolize progestins may decrease the plasma concentrations of progestins, and may decrease the effectiveness of progestin-only pills. Some drugs or herbal products that may decrease the effectiveness of progestin-only pills include:
- Barbiturates
- Bosentan
- Carbamazepine
- Felbamate
- Griseofulvin
- Oxcarbazepine
- Phenytoin
- Rifampin
- St. John’s wort
- Topiramate
Significant changes (increase or decrease) in the plasma levels of the progestin have been noted in some cases of coadministration with HIV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
Consult the labeling of all concurrently used drugs to obtain further information about interactions with progestin-only pills or the potential for enzyme alterations.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Many studies have found no harmful effects on fetal development associated with long-term use of contraceptive doses of oral progestins. The few studies of infant growth and development that have been conducted with progestin-only pills have not demonstrated significant adverse effects.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Levonorgestrel (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Levonorgestrel (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Levonorgestrel (oral) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Levonorgestrel (oral) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Levonorgestrel (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Levonorgestrel (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Levonorgestrel (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Levonorgestrel (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Levonorgestrel (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Levonorgestrel (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Levonorgestrel (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Levonorgestrel (oral) Administration in the drug label.
Monitoring
There is limited information regarding Levonorgestrel (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Levonorgestrel (oral) and IV administrations.
Overdosage
There is limited information regarding Levonorgestrel (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Levonorgestrel (oral) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Levonorgestrel (oral) Mechanism of Action in the drug label.
Structure
There is limited information regarding Levonorgestrel (oral) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Levonorgestrel (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Levonorgestrel (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Levonorgestrel (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Levonorgestrel (oral) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Levonorgestrel (oral) How Supplied in the drug label.
Storage
There is limited information regarding Levonorgestrel (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Levonorgestrel (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Levonorgestrel (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Levonorgestrel (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Levonorgestrel interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Levonorgestrel (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Levonorgestrel (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Implant; insert (extended-release); oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 55% |
| Metabolism | Hepatic |
| Elimination half-life | 36 ± 13 hours |
| Excretion | Renal: 45%; Fecal:32% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.446 g/mol |
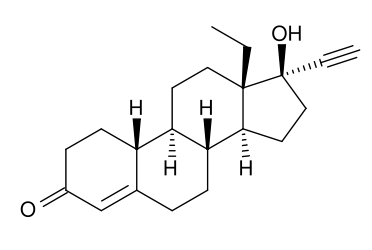
Levonorgestrel (or l-norgestrel or D-norgestrel) is a synthetic progestogen used as an active ingredient in some hormonal contraceptives.
Chemistry
Chemically, it is a hormonally active levorotatory enantiomer of the racemic mixture norgestrel. It is a gonane progestin derived from 19-nortestosterone.[1]
Its in vitro relative binding affinities at human steroid hormone receptors are: 323% that of progesterone at the progesterone receptor, 58% that of testosterone at the androgen receptor, 17% that of aldosterone at the mineralocorticoid receptor, 7.5% that of cortisol at the glucocorticoid receptor, and <0.02% that of estradiol at the estrogen receptor.[2]
Usage
Oral contraceptives
At low doses, levonorgestrel is used in monophasic and triphasic formulations of combined oral contraceptive pills, with available monophasic doses ranging from 100-250 µg, and triphasic doses of 50 µg/75 µg/125 µg.
At very low daily dose of 30 µg, levonorgestrel is used in some progestogen only pill formulations.
Emergency contraception
Levonorgestrel is used in emergency contraceptive pills (ECPs), both in a combined Yuzpe regimen which includes estrogen, and as a levonorgestrel-only method. The levonorgestrel-only method uses levonorgestrel 1500 μg (as a single dose or as two 750 μg doses 12 hours apart) taken within 3 days of unprotected sex. There are many brand names of levonorgestrel-only ECPs, including: Plan B, Levonelle, NorLevo, Postinor-2, and 72-HOURS.[3]
IUD
Levonorgestrel is the active ingredient in Mirena.
Contraceptive implants
Levonorgestrel is the active ingredient in Norplant and Jadelle.
References
- ↑ Edgren RA, Stanczyk FZ (1999). "Nomenclature of the gonane progestins". Contraception. 60 (6): 313. PMID 10715364.
- ↑ Sitruk-Ware R (2006). "New progestagens for contraceptive use". Hum Reprod Update. 12 (2): 169–78. PMID 16291771.
- ↑ Trussell, James; Cleland, Kelly (2007-04-10). "Emergency Contraceptive Pills Worldwide". Princeton University. Retrieved 2007-05-28.
External links
- Levonelle manufacturer's product information from Schering
- Monograph for levonorgestrel - Uk Medicines Information
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Hormonal contraception
- Progestagens
- Endocrinology