Ixazomib: Difference between revisions
No edit summary |
No edit summary |
||
| Line 108: | Line 108: | ||
|useInPregnancyFDA=Women should avoid becoming pregnant while being treated with NINLARO. | |useInPregnancyFDA=Women should avoid becoming pregnant while being treated with NINLARO. | ||
Risk Summary | '''Risk Summary''' | ||
NINLARO can cause fetal harm when administered to a pregnant woman. There are no human data available regarding the potential effect of NINLARO on pregnancy or development of the embryo or fetus. Ixazomib caused embryo-fetal toxicity in pregnant rats and rabbits at doses resulting in exposures that were slightly higher then those observed in patients receiving the recommended dose [see DATA]. Advise women of the potential risk to a fetus and to avoid becoming pregnant while being treated with NINLARO. | NINLARO can cause fetal harm when administered to a pregnant woman. There are no human data available regarding the potential effect of NINLARO on [[pregnancy]] or development of the embryo or fetus. Ixazomib caused embryo-fetal toxicity in pregnant rats and rabbits at doses resulting in exposures that were slightly higher then those observed in patients receiving the recommended dose [see DATA]. Advise women of the potential risk to a fetus and to avoid becoming pregnant while being treated with NINLARO. | ||
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. | In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. | ||
Data | '''Data''' | ||
Animal Data | '''Animal Data''' | ||
*In an embryo-fetal development study in pregnant rabbits there were increases in fetal skeletal variations/abnormalities (fused caudal vertebrae, number of [[lumbar vertebrae]], and full supernumerary ribs) at doses that were also maternally [[toxic]] (≥ 0.3 mg/kg). Exposures in the rabbit at 0.3 mg/kg were 1.9 times the clinical time averaged exposures at the recommended dose of 4 mg. In a rat dose range-finding embryo-fetal development study, at doses that were maternally toxic, there were decreases in fetal weights, a trend towards decreased fetal viability, and increased post-[[implantation]] losses at 0.6 mg/kg. Exposures in rats at the dose of 0.6 mg/kg was 2.5 times the clinical time averaged exposures at the recommended dose of 4 mg. | |||
In an embryo-fetal development study in pregnant rabbits there were increases in fetal skeletal variations/abnormalities (fused caudal vertebrae, number of lumbar vertebrae, and full supernumerary ribs) at doses that were also maternally toxic (≥ 0.3 mg/kg). Exposures in the rabbit at 0.3 mg/kg were 1.9 times the clinical time averaged exposures at the recommended dose of 4 mg. In a rat dose range-finding embryo-fetal development study, at doses that were maternally toxic, there were decreases in fetal weights, a trend towards decreased fetal viability, and increased post-implantation losses at 0.6 mg/kg. Exposures in rats at the dose of 0.6 mg/kg was 2.5 times the clinical time averaged exposures at the recommended dose of 4 mg. | |||
|useInPed=Safety and effectiveness have not been established in pediatric patients. | |useInPed=Safety and effectiveness have not been established in pediatric patients. | ||
|useInGeri=Of the total number of subjects in clinical studies of NINLARO, 55% were 65 and over, while 17% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |useInGeri=*Of the total number of subjects in clinical studies of NINLARO, 55% were 65 and over, while 17% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | ||
|useInRenalImpair=In patients with severe renal impairment or ESRD requiring dialysis, the mean AUC increased by 39% when compared to patients with normal renal function. Reduce the starting dose of NINLARO in patients with severe renal impairment or ESRD requiring dialysis. NINLARO is not dialyzable and therefore can be administered without regard to the timing of dialysis | |useInRenalImpair=*In patients with severe [[renal]] impairment or [[ESRD]] requiring [[dialysis]], the mean AUC increased by 39% when compared to patients with normal [[renal]] function. Reduce the starting dose of NINLARO in patients with severe [[renal]] impairment or ESRD requiring dialysis. NINLARO is not dialyzable and therefore can be administered without regard to the timing of [[dialysis]]. | ||
|useInHepaticImpair=In patients with moderate or severe hepatic impairment, the mean AUC increased by 20% when compared to patients with normal hepatic function. Reduce the starting dose of NINLARO in patients with moderate or severe hepatic impairment | |useInHepaticImpair=In patients with moderate or severe [[hepatic]] impairment, the mean AUC increased by 20% when compared to patients with normal [[hepatic]] function. Reduce the starting dose of NINLARO in patients with moderate or severe [[hepatic]] impairment. | ||
|useInReproPotential=Contraception | |useInReproPotential='''Contraception''' | ||
Male and female patients of childbearing potential must use effective contraceptive measures during and for 90 days following treatment. | *Male and female patients of childbearing potential must use effective [[contraceptive]] measures during and for 90 days following treatment. | ||
Infertility | '''Infertility''' | ||
*Fertility studies were not conducted with NINLARO; however there were no effects on reproductive organs in either males or females in nonclinical studies in rats and dogs | |||
Fertility studies were not conducted with NINLARO; however there were no effects on reproductive organs in either males or females in nonclinical studies in rats and dogs | |||
|othersTitle=Lactation | |othersTitle=Lactation | ||
|useInOthers=Risk Summary | |useInOthers='''Risk Summary''' | ||
*It is not known whether NINLARO or its metabolites are present in human milk. Many drugs are present in human milk and as a result, there could be a potential for adverse events in nursing infants. Advise women to discontinue nursing. | |||
It is not known whether NINLARO or its metabolites are present in human milk. Many drugs are present in human milk and as a result, there could be a potential for adverse events in nursing infants. Advise women to discontinue nursing. | |||
|administration=Please see adult indications and dosing | |administration=Please see adult indications and dosing | ||
|overdose=There is no known specific antidote for NINLARO overdose. In the event of an overdose, monitor the patient for adverse reactions | |overdose=*There is no known specific [[antidote]] for NINLARO [[overdose]]. In the event of an overdose, monitor the patient for adverse reactions and provide appropriate supportive care. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| drug_name = | | drug_name = | ||
| Line 191: | Line 188: | ||
| StdInChIKey = MXAYKZJJDUDWDS-LBPRGKRZSA-N | | StdInChIKey = MXAYKZJJDUDWDS-LBPRGKRZSA-N | ||
}} | }} | ||
|mechAction= | |mechAction=Ixazomib is a reversible proteasome inhibitor. Ixazomib preferentially binds and inhibits the chymotrypsin-like activity of the beta 5 subunit of the 20S proteasome. | ||
Chemical | Ixazomib induced apoptosis of multiple myeloma cell lines in vitro. Ixazomib demonstrated in vitro cytotoxicity against myeloma cells from patients who had relapsed after multiple prior therapies, including bortezomib, lenalidomide, and dexamethasone. The combination of ixazomib and lenalidomide demonstrated synergistic cytotoxic effects in multiple myeloma cell lines. In vivo, ixazomib demonstrated antitumor activity in a mouse multiple myeloma tumor xenograft model. | ||
|structure=[[File:Chemical structure 1655.PNG|thumb|center]] | |||
The molecular formula for ixazomib citrate is C20H23BCl2N2O9 and its molecular weight is 517.12. Ixazomib citrate has one chiral center and is the R-stereoisomer. The solubility of ixazomib citrate in 0.1N HCl (pH 1.2) at 37°C is 0.61 mg/mL (reported as ixazomib). The solubility increases as the pH increases. | The molecular formula for ixazomib citrate is C20H23BCl2N2O9 and its molecular weight is 517.12. Ixazomib citrate has one chiral center and is the R-stereoisomer. The solubility of ixazomib citrate in 0.1N HCl (pH 1.2) at 37°C is 0.61 mg/mL (reported as ixazomib). The solubility increases as the pH increases. | ||
*Ixazomib capsules for oral use contain 4, 3 or 2.3 mg of ixazomib equivalent to 5.7, 4.3 or 3.3 mg of ixazomib citrate, respectively. Inactive ingredients include microcrystalline cellulose, magnesium stearate, and talc. Capsule shells contain gelatin and titanium dioxide. The 4 mg capsule shell contains red and yellow iron oxide, the 3 mg capsule shell contains black iron oxide and the 2.3 mg capsule shell contains red iron oxide. The printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide. | |||
|PD=Cardiac Electrophysiology | |PD='''Cardiac Electrophysiology''' | ||
*NINLARO did not prolong the [[QTc]] interval at clinically relevant exposures based on [[pharmacokinetic]]-[[pharmacodynamic]] analysis of data from 245 patients. | |||
NINLARO did not prolong the QTc interval at clinically relevant exposures based on pharmacokinetic-pharmacodynamic analysis of data from 245 patients. | |PK='''Absorption''' | ||
|PK=Absorption | *After oral administration, the median time to achieve peak ixazomib [[plasma concentrations]] was one hour. The mean absolute oral [[bioavailability]] was 58%, based on population PK analysis. Ixazomib AUC increases in a dose proportional manner over a dose range of 0.2 to 10.6 mg. | ||
*A food effect study conducted in patients with a single 4 mg dose of ixazomib showed that a high-fat meal decreased ixazomib AUC by 28% and [[Cmax]] by 69%. | |||
After oral administration, the median time to achieve peak ixazomib plasma concentrations was one hour. The mean absolute oral bioavailability was 58%, based on population PK analysis. Ixazomib AUC increases in a dose proportional manner over a dose range of 0.2 to 10.6 mg. | '''Distribution''' | ||
*Ixazomib is 99% bound to [[plasma proteins]] and distributes into red blood cells with a blood-to-plasma ratio of 10. The steady-state [[volume of distribution]] is 543 L. | |||
A food effect study conducted in patients with a single 4 mg dose of ixazomib showed that a high-fat meal decreased ixazomib AUC by 28% and Cmax by 69% | |||
Distribution | |||
Ixazomib is 99% bound to plasma proteins and distributes into red blood cells with a blood-to-plasma ratio of 10. The steady-state volume of distribution is 543 L | |||
'''Elimination''' | |||
*Based on a population PK analysis, systemic clearance was approximately 1.9 L/hr with inter-individual variability of 44%. The terminal half-life ([[t1/2]]) of ixazomib was 9.5 days. Following weekly oral dosing, the accumulation ratio was determined to be 2-fold. | |||
'''Metabolism''' | |||
*After oral administration of a radiolabeled dose, ixazomib represented 70% of total drug-related material in plasma. Metabolism by multiple CYP enzymes and non-CYP proteins is expected to be the major clearance mechanism for ixazomib. At clinically relevant ixazomib concentrations, in vitro studies using human cDNA-expressed [[cytochrome P450]] isozymes showed that no specific CYP isozyme predominantly contributes to ixazomib metabolism. At higher than clinical concentrations, ixazomib was metabolized by multiple CYP isoforms with estimated relative contributions of 3A4 (42%), 1A2 (26%), 2B6 (16%), 2C8 (6%), 2D6 (5%), 2C19 (5%) and 2C9 (< 1%). | |||
'''Excretion''' | |||
*After administration of a single oral dose of 14C-ixazomib to 5 patients with advanced cancer, 62% of the administered radioactivity was excreted in urine and 22% in the [[feces]]. Unchanged ixazomib accounted for < 3.5% of the administered dose recovered in urine. | |||
'''Specific Populations''' | |||
'''Age, Sex, Race''' | |||
There was no clinically meaningful effect of age (range 23-91 years), sex, [[body surface area]](range 1.2-2.7 m2), or race on the clearance of ixazomib based on population PK analysis. | |||
'''Hepatic Impairment''' | |||
*The PK of ixazomib was similar in patients with normal [[hepatic]] function and in patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin > 1-1.5 × ULN and any AST) based on population PK analysis. | |||
The PK of ixazomib was characterized in patients with normal hepatic function at 4 mg (N=12), moderate hepatic impairment at 2.3 mg (total bilirubin > 1.5-3 × ULN, N=13) or severe hepatic impairment at 1.5 mg (total bilirubin > 3 × ULN, N=18). Dose-normalized mean AUC was 20% higher in patients with moderate or severe hepatic impairment as compared to patients with normal hepatic function. | |||
'''Renal Impairment''' | |||
*The PK of ixazomib was similar in patients with normal [[renal]] function and in patients with mild or moderate [[renal]] impairment ([[creatinine clearance]] ≥ 30 mL/min) based on population PK analysis. | |||
*The PK of ixazomib was characterized at a dose of 3 mg in patients with normal renal function ([[creatinine clearance]] ≥ 90 mL/min, N=18), severe renal impairment (creatinine clearance < 30 mL/min, N=14), or [[ESRD]] requiring dialysis (N=6). Mean AUC was 39% higher in patients with severe renal impairment or [[ESRD]] requiring [[dialysis]] as compared to patients with normal renal function. Pre- and post-dialyzer concentrations of ixazomib measured during the [[hemodialysis]] session were similar, suggesting that ixazomib is not [[dialyzable]]. | |||
'''Drug Interactions''' | |||
'''Effect of Other Drugs on NINLARO''' | |||
'''Strong CYP3A Inducers''' | |||
Co-administration of NINLARO with [[rifampin]] decreased ixazomib Cmax by 54% and AUC by 74%. | |||
'''Strong CYP3A Inhibitors''' | |||
*Co-administration of NINLARO with [[clarithromycin]] did not result in a clinically meaningful change in the systemic exposure of ixazomib. | |||
'''Strong CYP1A2 Inhibitor'''s | |||
*Co-administration of NINLARO with strong [[CYP1A2]] inhibitors did not result in a clinically meaningful change in the systemic exposure of ixazomib based on a population PK analysis. | |||
'''Effect of NINLARO on Other Drugs | |||
|nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility | ''' | ||
Ixazomib was not mutagenic in a bacterial reverse mutation assay (Ames assay). Ixazomib was considered positive in an in vitro clastogenicity test in human peripheral blood lymphocytes. However, in vivo, ixazomib was not clastogenic in a bone marrow micronucleus assay in mice and was negative in an in vivo comet assay in mice, as assessed in the stomach and liver. No carcinogenicity studies have been performed with ixazomib. | *Ixazomib is neither a reversible nor a time-dependent inhibitor of CYPs 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4/5. Ixazomib did not induce CYP1A2, CYP2B6, and CYP3A4/5 activity or corresponding immunoreactive protein levels. NINLARO is not expected to produce drug-drug interactions via CYP inhibition or induction. | ||
'''Transporter-Based Interactions''' | |||
*Ixazomib is a low affinity substrate of P-gp. Ixazomib is not a substrate of BCRP, MRP2 or hepatic OATPs. Ixazomib is not an inhibitor of P-gp, BCRP, MRP2, OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, or MATE2-K. NINLARO is not expected to cause transporter-mediated drug-drug interactions. | |||
|nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | |||
*Ixazomib was not mutagenic in a [[bacterial]] reverse [[mutation]] assay (Ames assay). Ixazomib was considered positive in an in vitro clastogenicity test in human peripheral blood lymphocytes. However, in vivo, ixazomib was not clastogenic in a [[bone marrow]] micronucleus assay in mice and was negative in an in vivo comet assay in mice, as assessed in the [[stomach]] and [[liver]]. No [[carcinogenicity]] studies have been performed with ixazomib. | |||
Developmental toxicity studies in rats and rabbits did not show direct embryo-fetal toxicity below maternally toxic doses of ixazomib. Studies of fertility and early embryonic development and pre- and postnatal toxicology were not conducted with ixazomib, but evaluation of reproductive tissues was conducted in the general toxicity studies. There were no effects due to ixazomib treatment on male or female reproductive organs in studies up to 6-months duration in rats and up to 9-months duration in dogs. | *Developmental toxicity studies in rats and rabbits did not show direct embryo-fetal toxicity below maternally toxic doses of ixazomib. Studies of [[fertility]] and early embryonic development and pre- and postnatal toxicology were not conducted with ixazomib, but evaluation of reproductive tissues was conducted in the general toxicity studies. There were no effects due to ixazomib treatment on male or female [[reproductive]] organs in studies up to 6-months duration in rats and up to 9-months duration in dogs. | ||
|clinicalStudies=The efficacy and safety of NINLARO in combination with lenalidomide and dexamethasone was evaluated in a randomized, double-blind, placebo-controlled, multicenter study in patients with relapsed and/or refractory multiple myeloma who had received at least one prior line of therapy. Patients who were refractory to lenalidomide or proteasome inhibitors were excluded from the study. | |clinicalStudies=The efficacy and safety of NINLARO in combination with lenalidomide and dexamethasone was evaluated in a randomized, double-blind, placebo-controlled, multicenter study in patients with relapsed and/or refractory multiple myeloma who had received at least one prior line of therapy. Patients who were refractory to lenalidomide or proteasome inhibitors were excluded from the study. | ||
Revision as of 16:43, 28 February 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vishal Devarkonda, M.B.B.S[2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ixazomib is a antineoplastic agent that is FDA approved for the treatment of indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.. Common adverse reactions include thrombocytopenia, gastrointestinal toxicities, peripheral neuropathy, peripheral edema, cutaneous reactions, and hepatotoxicity..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
NINLARO is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
Dosing and Administration Guidelines
- NINLARO is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least one prior therapy.
- The recommended starting dose of NINLARO is 4 mg administered orally once a week on Days 1, 8, and 15 of a 28-day treatment cycle.
- The recommended starting dose of lenalidomide is 25 mg administered daily on Days 1 through 21 of a 28-day treatment cycle.
- The recommended starting dose of dexamethasone is 40 mg administered on Days 1, 8, 15, and 22 of a 28-day treatment cycle.

For additional information regarding lenalidomide and dexamethasone, refer to their prescribing information.
- NINLARO should be taken once a week on the same day and at approximately the same time for the first three weeks of a four week cycle. NINLARO should be taken at least one hour before or at least two hours after food. The whole capsule should be swallowed with water. The capsule should not be crushed, chewed or opened.
- If a NINLARO dose is delayed or missed, the dose should be taken only if the next scheduled dose is ≥ 72 hours away. A missed dose should not be taken within 72 hours of the next scheduled dose. A double dose should not be taken to make up for the missed dose.
- If vomiting occurs after taking a dose, the patient should not repeat the dose. The patient should resume dosing at the time of the next scheduled dose.
Prior to initiating a new cycle of therapy:
Absolute neutrophil count should be at least 1,000/mm3 Platelet count should be at least 75,000/mm3 Non-hematologic toxicities should, at the physician's discretion, generally be recovered to patient's baseline condition or Grade 1 or lower Treatment should be continued until disease progression or unacceptable toxicity.
Dose Modification Guidelines
- The NINLARO dose reduction steps are presented in Table 2 and the dose modification guidelines are provided in Table 3.


An alternating dose modification approach is recommended for NINLARO and lenalidomide for thrombocytopenia, neutropenia, and rash as described in Table 3. Refer to the lenalidomide prescribing information if dose reduction is needed for lenalidomide. Dosage in Patients with Hepatic Impairment
- Reduce the starting dose of NINLARO to 3 mg in patients with moderate (total bilirubin greater than 1.5-3 × ULN) or severe (total bilirubin greater than 3 × ULN) hepatic impairment.
Dosage in Patients with Renal Impairment
- Reduce the starting dose of NINLARO to 3 mg in patients with severe renal impairment (creatinine clearance less than 30 mL/min) or end-stage renal disease (ESRD) requiring dialysis. NINLARO is not dialyzable and therefore can be administered without regard to the timing of dialysis.
- Refer to the lenalidomide prescribing information for dosing recommendations in patients with renal impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ixazomib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ixazomib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ixazomib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ixazomib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ixazomib in pediatric patients.
Contraindications
None.
Warnings
Thrombocytopenia
- Thrombocytopenia has been reported with NINLARO with platelet nadirs typically occurring between Days 14-21 of each 28-day cycle and recovery to baseline by the start of the next cycle. Three percent of patients in the NINLARO regimen and 1% of patients in the placebo regimen had a platelet count ≤ 10,000/mm3 during treatment. Less than 1% of patients in both regimens had a platelet count ≤ 5000/mm3 during treatment. Discontinuations due to thrombocytopenia were similar in both regimens (< 1% of patients in the NINLARO regimen and 2% of patients in the placebo regimen discontinued one or more of the three drugs).The rate of platelet transfusions was 6% in the NINLARO regimen and 5% in the placebo regimen.
- Monitor platelet counts at least monthly during treatment with NINLARO. Consider more frequent monitoring during the first three cycles. Manage thrombocytopenia with dose modifications and platelet transfusions as per standard medical guidelines.
Gastrointestinal Toxicities
- Diarrhea, constipation, nausea, and vomiting, have been reported with NINLARO, occasionally requiring use of antidiarrheal and antiemetic medications, and supportive care. Diarrhea was reported in 42% of patients in the NINLARO regimen and 36% in the placebo regimen, constipation in 34% and 25%, respectively, nausea in 26% and 21%, respectively, and vomiting in 22% and 11%, respectively. Diarrhea resulted in discontinuation of one or more of the three drugs in 1% of patients in the NINLARO regimen and < 1% of patients in the placebo regimen. Adjust dosing for Grade 3 or 4 symptoms.
Peripheral Neuropathy
- The majority of peripheral neuropathy adverse reactions were Grade 1 (18% in the NINLARO regimen and 14% in the placebo regimen) and Grade 2 (8% in the NINLARO regimen and 5% in the placebo regimen). Grade 3 adverse reactions of peripheral neuropathy were reported at 2% in both regimens; there were no Grade 4 or serious adverse reactions.
- The most commonly reported reaction was peripheral sensory neuropathy (19% and 14% in the NINLARO and placebo regimen, respectively). Peripheral motor neuropathy was not commonly reported in either regimen (< 1%). Peripheral neuropathy resulted in discontinuation of one or more of the three drugs in 1% of patients in both regimens. Patients should be monitored for symptoms of neuropathy. Patients experiencing new or worsening peripheral neuropathy may require dose modification.
Peripheral Edema
- Peripheral edema was reported in 25% and 18% of patients in the NINLARO and placebo regimens, respectively. The majority of peripheral edema adverse reactions were Grade 1 (16% in the NINLARO regimen and 13% in the placebo regimen) and Grade 2 (7% in the NINLARO regimen and 4% in the placebo regimen).
- Grade 3 peripheral edema was reported in 2% and 1% of patients in the NINLARO and placebo regimens, respectively. There was no Grade 4 peripheral edema reported. There were no discontinuations reported due to peripheral edema. Evaluate for underlying causes and provide supportive care, as necessary. Adjust dosing of dexamethasone per its prescribing information or NINLARO for Grade 3 or 4 symptoms.
Cutaneous Reactions
- Rash was reported in 19% of patients in the NINLARO regimen and 11% of patients in the placebo regimen. The majority of the rash adverse reactions were Grade 1 (10% in the NINLARO regimen and 7% in the placebo regimen) or Grade 2 (6% in the NINLARO regimen and 3% in the placebo regimen). Grade 3 rash was reported in 3% of patients in the NINLARO regimen and 1% of patients in the placebo regimen. There were no Grade 4 or serious adverse reactions of rash reported. The most common type of rash reported in both regimens included maculo-papular and macular rash. Rash resulted in discontinuation of one or more of the three drugs in < 1% of patients in both regimens. Manage rash with supportive care or with dose modification if Grade 2 or higher.
Hepatotoxicity
- Drug-induced liver injury, hepatocellular injury, hepatic steatosis, hepatitis cholestatic and hepatotoxicity have each been reported in < 1% of patients treated with NINLARO. Events of liver impairment have been reported (6% in the NINLARO regimen and 5% in the placebo regimen). Monitor hepatic enzymes regularly and adjust dosing for Grade 3 or 4 symptoms.
Embryo-Fetal Toxicity
- NINLARO can cause fetal harm when administered to a pregnant woman based on the mechanism of action and findings in animals. There are no adequate and well-controlled studies in pregnant women using NINLARO. Ixazomib caused embryo-fetal toxicity in pregnant rats and rabbits at doses resulting in exposures that were slightly higher than those observed in patients receiving the recommended dose.
- Females of reproductive potential should be advised to avoid becoming pregnant while being treated with NINLARO. If NINLARO is used during pregnancy or if the patient becomes pregnant while taking NINLARO, the patient should be apprised of the potential hazard to the fetus. Advise females of reproductive potential that they must use effective contraception during treatment with NINLARO and for 90 days following the final dose.
Adverse Reactions
Clinical Trials Experience
- Thrombocytopenia
- Gastrointestinal Toxicities
- Peripheral Neuropathy
- Peripheral Edema
- Cutaneous Reactions
- Hepatotoxicity
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety population from the randomized, double-blind, placebo-controlled clinical study included 720 patients with relapsed and/or refractory multiple myeloma, who received NINLARO in combination with lenalidomide and dexamethasone (NINLARO regimen; N=360) or placebo in combination with lenalidomide and dexamethasone (placebo regimen; N=360).
- The most frequently reported adverse reactions (≥ 20%) in the NINLARO regimen and greater than the placebo regimen were diarrhea, constipation, thrombocytopenia, peripheral neuropathy, nausea, peripheral edema, vomiting, and back pain. Serious adverse reactions reported in ≥ 2% of patients included thrombocytopenia (2%) and diarrhea (2%). For each adverse reaction, one or more of the three drugs was discontinued in ≤ 1% of patients in the NINLARO regimen.
- Table 4 summarizes the adverse reactions occurring in at least 5% of patients with at least a 5% difference between the NINLARO regimen and the placebo regimen.

.

Eye Disorders
- Eye disorders were reported with many different preferred terms but in aggregate, the frequency was 26% in patients in the NINLARO regimen and 16% of patients in the placebo regimen. The most common adverse reactions were blurred vision (6% in the NINLARO regimen and 3% in the placebo regimen), dry eye (5% in the NINLARO regimen and 1% in the placebo regimen), and conjunctivitis (6% in the NINLARO regimen and 1% in the placebo regimen). Grade 3 adverse reactions were reported in 2% of patients in the NINLARO regimen and 1% in the placebo regimen.
Adverse Reactions Reported Outside of the Randomized Controlled Trial
- The following serious adverse reactions have each been reported at a frequency of < 1%: acute febrile neutrophilic dermatosis (Sweet's syndrome), Stevens-Johnson syndrome, transverse myelitis, posterior reversible encephalopathy syndrome, tumor lysis syndrome, and thrombotic thrombocytopenic purpura.
Postmarketing Experience
There is limited information regarding Ixazomib Postmarketing Experience in the drug label.
Drug Interactions
Strong CYP3A Inducers Avoid concomitant administration of NINLARO with strong CYP3A inducers (such as rifampin, phenytoin, carbamazepine, and St. John's Wort)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Women should avoid becoming pregnant while being treated with NINLARO.
Risk Summary
NINLARO can cause fetal harm when administered to a pregnant woman. There are no human data available regarding the potential effect of NINLARO on pregnancy or development of the embryo or fetus. Ixazomib caused embryo-fetal toxicity in pregnant rats and rabbits at doses resulting in exposures that were slightly higher then those observed in patients receiving the recommended dose [see DATA]. Advise women of the potential risk to a fetus and to avoid becoming pregnant while being treated with NINLARO.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
- In an embryo-fetal development study in pregnant rabbits there were increases in fetal skeletal variations/abnormalities (fused caudal vertebrae, number of lumbar vertebrae, and full supernumerary ribs) at doses that were also maternally toxic (≥ 0.3 mg/kg). Exposures in the rabbit at 0.3 mg/kg were 1.9 times the clinical time averaged exposures at the recommended dose of 4 mg. In a rat dose range-finding embryo-fetal development study, at doses that were maternally toxic, there were decreases in fetal weights, a trend towards decreased fetal viability, and increased post-implantation losses at 0.6 mg/kg. Exposures in rats at the dose of 0.6 mg/kg was 2.5 times the clinical time averaged exposures at the recommended dose of 4 mg.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ixazomib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ixazomib during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Ixazomib in women who are nursing.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Geriatic Use
- Of the total number of subjects in clinical studies of NINLARO, 55% were 65 and over, while 17% were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Ixazomib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ixazomib with respect to specific racial populations.
Renal Impairment
- In patients with severe renal impairment or ESRD requiring dialysis, the mean AUC increased by 39% when compared to patients with normal renal function. Reduce the starting dose of NINLARO in patients with severe renal impairment or ESRD requiring dialysis. NINLARO is not dialyzable and therefore can be administered without regard to the timing of dialysis.
Hepatic Impairment
In patients with moderate or severe hepatic impairment, the mean AUC increased by 20% when compared to patients with normal hepatic function. Reduce the starting dose of NINLARO in patients with moderate or severe hepatic impairment.
Females of Reproductive Potential and Males
Contraception
- Male and female patients of childbearing potential must use effective contraceptive measures during and for 90 days following treatment.
Infertility
- Fertility studies were not conducted with NINLARO; however there were no effects on reproductive organs in either males or females in nonclinical studies in rats and dogs
Immunocompromised Patients
There is no FDA guidance one the use of Ixazomib in patients who are immunocompromised.
Lactation
Risk Summary
- It is not known whether NINLARO or its metabolites are present in human milk. Many drugs are present in human milk and as a result, there could be a potential for adverse events in nursing infants. Advise women to discontinue nursing.
Administration and Monitoring
Administration
Please see adult indications and dosing
Monitoring
There is limited information regarding Ixazomib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ixazomib and IV administrations.
Overdosage
- There is no known specific antidote for NINLARO overdose. In the event of an overdose, monitor the patient for adverse reactions and provide appropriate supportive care.
Pharmacology
Mechanism of Action
Ixazomib is a reversible proteasome inhibitor. Ixazomib preferentially binds and inhibits the chymotrypsin-like activity of the beta 5 subunit of the 20S proteasome.
Ixazomib induced apoptosis of multiple myeloma cell lines in vitro. Ixazomib demonstrated in vitro cytotoxicity against myeloma cells from patients who had relapsed after multiple prior therapies, including bortezomib, lenalidomide, and dexamethasone. The combination of ixazomib and lenalidomide demonstrated synergistic cytotoxic effects in multiple myeloma cell lines. In vivo, ixazomib demonstrated antitumor activity in a mouse multiple myeloma tumor xenograft model.
Structure

The molecular formula for ixazomib citrate is C20H23BCl2N2O9 and its molecular weight is 517.12. Ixazomib citrate has one chiral center and is the R-stereoisomer. The solubility of ixazomib citrate in 0.1N HCl (pH 1.2) at 37°C is 0.61 mg/mL (reported as ixazomib). The solubility increases as the pH increases.
- Ixazomib capsules for oral use contain 4, 3 or 2.3 mg of ixazomib equivalent to 5.7, 4.3 or 3.3 mg of ixazomib citrate, respectively. Inactive ingredients include microcrystalline cellulose, magnesium stearate, and talc. Capsule shells contain gelatin and titanium dioxide. The 4 mg capsule shell contains red and yellow iron oxide, the 3 mg capsule shell contains black iron oxide and the 2.3 mg capsule shell contains red iron oxide. The printing ink contains shellac, propylene glycol, potassium hydroxide, and black iron oxide.
Pharmacodynamics
Cardiac Electrophysiology
- NINLARO did not prolong the QTc interval at clinically relevant exposures based on pharmacokinetic-pharmacodynamic analysis of data from 245 patients.
Pharmacokinetics
Absorption
- After oral administration, the median time to achieve peak ixazomib plasma concentrations was one hour. The mean absolute oral bioavailability was 58%, based on population PK analysis. Ixazomib AUC increases in a dose proportional manner over a dose range of 0.2 to 10.6 mg.
- A food effect study conducted in patients with a single 4 mg dose of ixazomib showed that a high-fat meal decreased ixazomib AUC by 28% and Cmax by 69%.
Distribution
- Ixazomib is 99% bound to plasma proteins and distributes into red blood cells with a blood-to-plasma ratio of 10. The steady-state volume of distribution is 543 L.
Elimination
- Based on a population PK analysis, systemic clearance was approximately 1.9 L/hr with inter-individual variability of 44%. The terminal half-life (t1/2) of ixazomib was 9.5 days. Following weekly oral dosing, the accumulation ratio was determined to be 2-fold.
Metabolism
- After oral administration of a radiolabeled dose, ixazomib represented 70% of total drug-related material in plasma. Metabolism by multiple CYP enzymes and non-CYP proteins is expected to be the major clearance mechanism for ixazomib. At clinically relevant ixazomib concentrations, in vitro studies using human cDNA-expressed cytochrome P450 isozymes showed that no specific CYP isozyme predominantly contributes to ixazomib metabolism. At higher than clinical concentrations, ixazomib was metabolized by multiple CYP isoforms with estimated relative contributions of 3A4 (42%), 1A2 (26%), 2B6 (16%), 2C8 (6%), 2D6 (5%), 2C19 (5%) and 2C9 (< 1%).
Excretion
- After administration of a single oral dose of 14C-ixazomib to 5 patients with advanced cancer, 62% of the administered radioactivity was excreted in urine and 22% in the feces. Unchanged ixazomib accounted for < 3.5% of the administered dose recovered in urine.
Specific Populations
Age, Sex, Race
There was no clinically meaningful effect of age (range 23-91 years), sex, body surface area(range 1.2-2.7 m2), or race on the clearance of ixazomib based on population PK analysis.
Hepatic Impairment
- The PK of ixazomib was similar in patients with normal hepatic function and in patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin > 1-1.5 × ULN and any AST) based on population PK analysis.
The PK of ixazomib was characterized in patients with normal hepatic function at 4 mg (N=12), moderate hepatic impairment at 2.3 mg (total bilirubin > 1.5-3 × ULN, N=13) or severe hepatic impairment at 1.5 mg (total bilirubin > 3 × ULN, N=18). Dose-normalized mean AUC was 20% higher in patients with moderate or severe hepatic impairment as compared to patients with normal hepatic function. Renal Impairment
- The PK of ixazomib was similar in patients with normal renal function and in patients with mild or moderate renal impairment (creatinine clearance ≥ 30 mL/min) based on population PK analysis.
- The PK of ixazomib was characterized at a dose of 3 mg in patients with normal renal function (creatinine clearance ≥ 90 mL/min, N=18), severe renal impairment (creatinine clearance < 30 mL/min, N=14), or ESRD requiring dialysis (N=6). Mean AUC was 39% higher in patients with severe renal impairment or ESRD requiring dialysis as compared to patients with normal renal function. Pre- and post-dialyzer concentrations of ixazomib measured during the hemodialysis session were similar, suggesting that ixazomib is not dialyzable.
Drug Interactions
Effect of Other Drugs on NINLARO
Strong CYP3A Inducers
Co-administration of NINLARO with rifampin decreased ixazomib Cmax by 54% and AUC by 74%. Strong CYP3A Inhibitors
- Co-administration of NINLARO with clarithromycin did not result in a clinically meaningful change in the systemic exposure of ixazomib.
Strong CYP1A2 Inhibitors
- Co-administration of NINLARO with strong CYP1A2 inhibitors did not result in a clinically meaningful change in the systemic exposure of ixazomib based on a population PK analysis.
Effect of NINLARO on Other Drugs
- Ixazomib is neither a reversible nor a time-dependent inhibitor of CYPs 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, or 3A4/5. Ixazomib did not induce CYP1A2, CYP2B6, and CYP3A4/5 activity or corresponding immunoreactive protein levels. NINLARO is not expected to produce drug-drug interactions via CYP inhibition or induction.
Transporter-Based Interactions
- Ixazomib is a low affinity substrate of P-gp. Ixazomib is not a substrate of BCRP, MRP2 or hepatic OATPs. Ixazomib is not an inhibitor of P-gp, BCRP, MRP2, OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, or MATE2-K. NINLARO is not expected to cause transporter-mediated drug-drug interactions.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Ixazomib was not mutagenic in a bacterial reverse mutation assay (Ames assay). Ixazomib was considered positive in an in vitro clastogenicity test in human peripheral blood lymphocytes. However, in vivo, ixazomib was not clastogenic in a bone marrow micronucleus assay in mice and was negative in an in vivo comet assay in mice, as assessed in the stomach and liver. No carcinogenicity studies have been performed with ixazomib.
- Developmental toxicity studies in rats and rabbits did not show direct embryo-fetal toxicity below maternally toxic doses of ixazomib. Studies of fertility and early embryonic development and pre- and postnatal toxicology were not conducted with ixazomib, but evaluation of reproductive tissues was conducted in the general toxicity studies. There were no effects due to ixazomib treatment on male or female reproductive organs in studies up to 6-months duration in rats and up to 9-months duration in dogs.
Clinical Studies
The efficacy and safety of NINLARO in combination with lenalidomide and dexamethasone was evaluated in a randomized, double-blind, placebo-controlled, multicenter study in patients with relapsed and/or refractory multiple myeloma who had received at least one prior line of therapy. Patients who were refractory to lenalidomide or proteasome inhibitors were excluded from the study.
A total of 722 patients were randomized in a 1:1 ratio to receive either the combination of NINLARO, lenalidomide and dexamethasone (N=360; NINLARO regimen) or the combination of placebo, lenalidomide and dexamethasone (N=362; placebo regimen) until disease progression or unacceptable toxicity. Randomization was stratified according to number of prior lines of therapy (1 versus 2 or 3), myeloma International Staging System (ISS) (stage I or II versus III), and previous therapy with a proteasome inhibitor (exposed or naïve). Twenty three percent (N=166) of the patients had light chain disease and 12% (N=87) of patients had free light chain-measurable only disease.
Thromboprophylaxis was recommended for all patients in both treatment groups according to the lenalidomide prescribing information. Antiemetics were used in 19% of patients in the NINLARO regimen and 12% of patients in the placebo regimen; antivirals in 64% and 60%, respectively, and antihistamines in 27% and 19%, respectively. These medications were given to patients at the physician's discretion as prophylaxis and/or management of symptoms.
Patients received NINLARO 4 mg or placebo on Days 1, 8, and 15 plus lenalidomide (25 mg) on Days 1 through 21 and dexamethasone (40 mg) on Days 1, 8, 15, and 22 of a 28-day cycle. Patients with renal impairment received a starting dose of lenalidomide according to its prescribing information. Treatment continued until disease progression or unacceptable toxicities.
Table 6 summarizes the baseline patient and disease characteristics in the study. The baseline demographics and disease characteristics were balanced and comparable between the study regimens.

The efficacy of NINLARO was evaluated by progression-free survival (PFS) according to the 2011 International Myeloma Working Group (IMWG) Consensus Uniform Response Criteria as assessed by a blinded independent review committee (IRC) based on central lab results. Response was assessed every four weeks until disease progression.
The approval of NINLARO was based upon a statistically significant improvement in PFS of the NINLARO regimen compared to the placebo regimen. PFS results are summarized in Table 7 and shown in Figure 1.
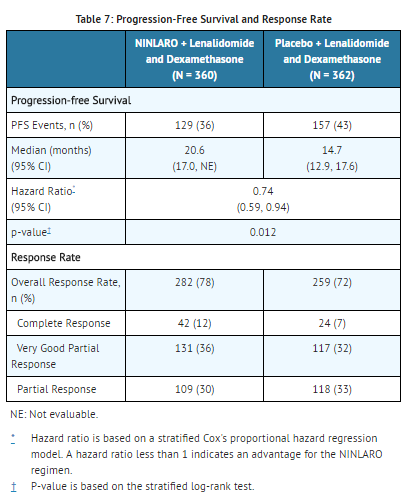
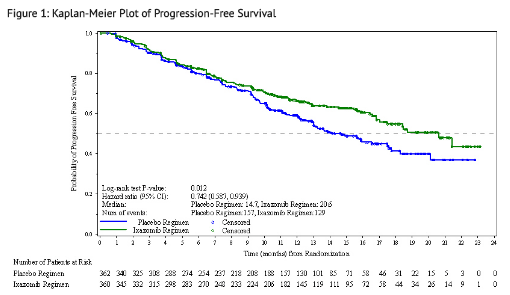
A non-inferential PFS analysis was conducted at a median follow up of 23 months with 372 PFS events. Hazard ratio of PFS was 0.82 (95% confidence interval [0.67, 1.0]) for NINLARO regimen versus placebo regimen, and estimated median PFS was 20 months in the NINLARO regimen and 15.9 months in the placebo regimen. At the same time, a planned interim OS analysis was conducted with 35% of the required number of deaths for final OS analysis; there were 81 deaths in the NINLARO regimen and 90 deaths in the placebo regimen. An OS benefit was not demonstrated.
How Supplied
A non-inferential PFS analysis was conducted at a median follow up of 23 months with 372 PFS events. Hazard ratio of PFS was 0.82 (95% confidence interval [0.67, 1.0]) for NINLARO regimen versus placebo regimen, and estimated median PFS was 20 months in the NINLARO regimen and 15.9 months in the placebo regimen. At the same time, a planned interim OS analysis was conducted with 35% of the required number of deaths for final OS analysis; there were 81 deaths in the NINLARO regimen and 90 deaths in the placebo regimen. An OS benefit was not demonstrated.
Storage
A non-inferential PFS analysis was conducted at a median follow up of 23 months with 372 PFS events. Hazard ratio of PFS was 0.82 (95% confidence interval [0.67, 1.0]) for NINLARO regimen versus placebo regimen, and estimated median PFS was 20 months in the NINLARO regimen and 15.9 months in the placebo regimen. At the same time, a planned interim OS analysis was conducted with 35% of the required number of deaths for final OS analysis; there were 81 deaths in the NINLARO regimen and 90 deaths in the placebo regimen. An OS benefit was not demonstrated.
16.3 Handling and Disposal NINLARO is cytotoxic1. Capsules should not be opened or crushed. Direct contact with the capsule contents should be avoided. In case of capsule breakage, avoid direct contact of capsule contents with the skin or eyes. If contact occurs with the skin, wash thoroughly with soap and water. If contact occurs with the eyes, flush thoroughly with water.
Any unused medicinal product or waste material should be disposed in accordance with local requirements.
Images
Drug Images
{{#ask: Page Name::Ixazomib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ixazomib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Dosing Instructions
Instruct patients to take NINLARO exactly as prescribed. Advise patients to take NINLARO once a week on the same day and at approximately the same time for the first three weeks of a four week cycle. Advise patients to take NINLARO at least one hour before or at least two hours after food. Advise patients that NINLARO and dexamethasone should not be taken at the same time, because dexamethasone should be taken with food and NINLARO should not be taken with food. Advise patients to swallow the capsule whole with water. The capsule should not be crushed, chewed or opened. Advise patients that direct contact with the capsule contents should be avoided. In case of capsule breakage, avoid direct contact of capsule contents with the skin or eyes. If contact occurs with the skin, wash thoroughly with soap and water. If contact occurs with the eyes, flush thoroughly with water. If a patient misses a dose, advise them to take the missed dose as long as the next scheduled dose is ≥ 72 hours away. Advise patients not to take a missed dose if it is within 72 hours of their next scheduled dose. If a patient vomits after taking a dose, advise them not to repeat the dose but resume dosing at the time of the next scheduled dose. Advise patients to store capsules in original packaging, and not to remove the capsule from the packaging until just prior to taking NINLARO. [see DOSAGE AND ADMINISTRATION (2.1)]
Thrombocytopenia
Advise patients that they may experience low platelet counts (thrombocytopenia). Signs of thrombocytopenia may include bleeding and easy bruising. [see WARNINGS AND PRECAUTIONS (5.1)].
Gastrointestinal Toxicities
Advise patients they may experience diarrhea, constipation, nausea and vomiting and to contact their physician if these adverse reactions persist. [see WARNINGS AND PRECAUTIONS (5.2)].
Peripheral Neuropathy
Advise patients to contact their physicians if they experience new or worsening symptoms of peripheral neuropathy such as tingling, numbness, pain, a burning feeling in the feet or hands, or weakness in the arms or legs. [see WARNINGS AND PRECAUTIONS (5.3)].
Peripheral Edema
Advise patients to contact their physicians if they experience unusual swelling of their extremities or weight gain due to swelling [see WARNINGS AND PRECAUTIONS (5.4)].
Cutaneous Reactions
Advise patients to contact their physicians if they experience new or worsening rash [see WARNINGS AND PRECAUTIONS (5.5)].
Hepatotoxicity
Advise patients to contact their physicians if they experience jaundice or right upper quadrant abdominal pain [see WARNINGS AND PRECAUTIONS (5.6)].
Pregnancy
Advise women of the potential risk to a fetus and to avoid becoming pregnant while being treated with NINLARO and for 90 days following the final dose. Advise patients to contact their physicians immediately if they or their female partner become pregnant during treatment or within 90 days of the final dose [see WARNINGS AND PRECAUTIONS (5.7)].
Concomitant Medications
Advise patients to speak with their physicians about any other medication they are currently taking and before starting any new medications.
Precautions with Alcohol
Alcohol-Ixazomib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ixazomib Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ixazomib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Ninlaro (ixazomib) Capsules, for Oral Use. Full Prescribing Information" (PDF). Ninlaro (ixazomib) For Healthcare Professionals. Takeda Pharmaceutical Company Limited. Retrieved 21 November 2015.