Idarubicin hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Idarubicin hydrochloride is an anthracycline and antineoplastic agent that is FDA approved for the treatment of acute myeloid leukemia (AML) in adults. This includes French-American-British (FAB) classifications M1 through M7. Common adverse reactions include alopecia, rash, urticaria, diarrhea, nausea, vomiting, stomach cramps and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Idarubicin hydrochloride FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Idarubicin hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Idarubicin hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Idarubicin hydrochloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Idarubicin hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Idarubicin hydrochloride in pediatric patients.
Contraindications
There is limited information regarding Idarubicin hydrochloride Contraindications in the drug label.
Warnings
- Idarubicin is intended for administration under the supervision of a physician who is experienced in leukemia chemotherapy.
- Idarubicin is a potent bone marrow suppressant. Idarubicin should not be given to patients with pre-existing bone marrow suppression induced by previous drug therapy or radiotherapy unless the benefit warrants the risk.
- Severe myelosuppression will occur in all patients given a therapeutic dose of this agent for induction, consolidation or maintenance. Careful hematologic monitoring is required. Deaths due to infection and/or bleeding have been reported during the period of severe myelosuppression. Facilities with laboratory and supportive resources adequate to monitor drug tolerability and protect and maintain a patient compromised by drug toxicity should be available. It must be possible to treat rapidly and completely a severe hemorrhagic condition and/or a severe infection.
- Pre-existing heart disease and previous therapy with anthracyclines at high cumulative doses or other potentially cardiotoxic agents are co-factors for increased risk of idarubicin-induced cardiac toxicity and the benefit to risk ratio of idarubicin therapy in such patients should be weighed before starting treatment with idarubicin.
- Myocardial toxicity as manifested by potentially fatal congestive heart failure, acute life-threatening arrhythmias or other cardiomyopathies may occur following therapy with idarubicin. Appropriate therapeutic measures for the management of congestive heart failure and/or arrhythmias are indicated.
- Cardiac function should be carefully monitored during treatment in order to minimize the risk of cardiac toxicity of the type described for other anthracycline compounds. The risk of such myocardial toxicity may be higher following concomitant or previous radiation to the mediastinal-pericardial area or in patients with anemia, bone marrow depression, infections, leukemic pericarditis and/or myocarditis, active or dormant cardiovascular disease, previous therapy with other anthracyclines or anthracenediones, and concomitant use of drugs with the ability to suppress cardiac contractility or cardiotoxic drugs (e.g., trastuzumab, cyclophosphamide and paclitaxel). Do not administer idarubicin with other cardiotoxic agents unless the patient's cardiac function is monitored frequently. Patients receiving anthracyclines after stopping treatment with other cardiotoxic agents, especially those with long half-lives, may also be at an increased risk of developing cardiotoxicity. Avoid the use of anthracycline-based therapy for at least 5 half-lives after discontinuation of the cardiotoxic agent. If anthracyclines are used before this time, carefully monitor the cardiac function. While there are no reliable means for predicting congestive heart failure, cardiomyopathy induced by anthracyclines is usually associated with a decrease of the left ventricular ejection fraction (LVEF) from pretreatment baseline values.
- Since hepatic impairment and/or renal function impairment can affect the disposition of idarubicin, liver and kidney function should be evaluated with conventional clinical laboratory tests (using serum bilirubin and serum creatinine as indicators) prior to and during treatment. In a number of Phase III clinical trials, treatment was not given if bilirubin and/or creatinine serum levels exceeded 2 mg%. However, in one Phase III trial, patients with bilirubin levels between 2.6 and 5 mg% received the anthracycline with a 50% reduction in dose. Dose reduction of idarubicin should be considered if the bilirubin and/or creatinine levels are above the normal range.
Adverse Reactions
Clinical Trials Experience
- Approximately 550 patients with AML have received idarubicin in combination with cytarabine in controlled clinical trials worldwide. In addition, over 550 patients with acute leukemia have been treated in uncontrolled trials utilizing idarubicin as a single agent or in combination. The table below lists the adverse experiences reported in U.S.
- Study 2 and is representative of the experiences in other studies. These adverse experiences constitute all reported or observed experiences, including those not considered to be drug related. Patients undergoing induction therapy for AML are seriously ill due to their disease, are receiving multiple transfusions, and concomitant medications including potentially toxic antibiotics and antifungal agents. The contribution of the study drug to the adverse experience profile is difficult to establish.
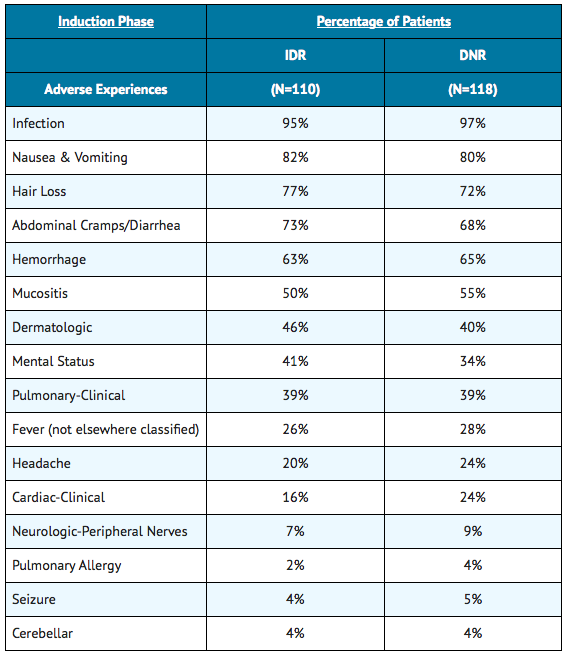
The duration of aplasia and incidence of mucositis were greater on the IDR arm than the DNR arm, especially during consolidation in some U.S. controlled trials.
The following information reflects experience based on U.S. controlled clinical trials.
Myelosuppression
- Severe myelosuppression is the major toxicity associated with idarubicin therapy, but this effect of the drug is required in order to eradicate the leukemic clone. During the period of myelosuppression, patients are at risk of developing infection and bleeding which may be life-threatening or fatal.
Gastrointestinal
- Nausea and/or vomiting, mucositis, abdominal pain and diarrhea were reported frequently, but were severe (equivalent to WHO Grade 4) in less than 5% of patients. Severe enterocolitis with perforation has been reported rarely. The risk of perforation may be increased by instrumental intervention. The possibility of perforation should be considered in patients who develop severe abdominal pain and appropriate steps for diagnosis and management should be taken.
Dermatologic
- Alopecia was reported frequently and dermatologic reactions including generalized rash, urticaria and a bullous erythrodermatous rash of the palms and soles have occurred. The dermatologic reactions were usually attributed to concomitant antibiotic therapy. Local reactions including hives at the injection site have been reported. Recall of skin reaction due to prior radiotherapy has occurred with idarubicin administration.
Hepatic and Renal
- Changes in hepatic and renal function tests have been observed. These changes were usually transient and occurred in the setting of sepsis and while patients were receiving potentially hepatotoxic and nephrotoxic antibiotics and antifungal agents. Severe changes in renal function (equivalent to WHO Grade 4) occurred in no more than 1% of patients, while severe changes in hepatic function (equivalent to WHO Grade 4) occurred in less than 5% of patients.
Cardiac
- Congestive heart failure (frequently attributed to fluid overload), serious arrhythmias including atrial fibrillation, chest pain, myocardial infarction and asymptomatic declines in LVEF have been reported in patients undergoing induction therapy for AML. Myocardial insufficiency and arrhythmias were usually reversible and occurred in the setting of sepsis, anemia and aggressive intravenous fluid administration. The events were reported more frequently in patients over age 60 years and in those with pre-existing cardiac disease.
Postmarketing Experience
There is limited information regarding Idarubicin hydrochloride Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Idarubicin hydrochloride Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Idarubicin hydrochloride in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Idarubicin hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Idarubicin hydrochloride during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Idarubicin hydrochloride in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Idarubicin hydrochloride in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Idarubicin hydrochloride in geriatric settings.
Gender
There is no FDA guidance on the use of Idarubicin hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Idarubicin hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Idarubicin hydrochloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Idarubicin hydrochloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Idarubicin hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Idarubicin hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Idarubicin hydrochloride Administration in the drug label.
Monitoring
There is limited information regarding Idarubicin hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Idarubicin hydrochloride and IV administrations.
Overdosage
There is limited information regarding Idarubicin hydrochloride overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Idarubicin hydrochloride Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Idarubicin hydrochloride Mechanism of Action in the drug label.
Structure
There is limited information regarding Idarubicin hydrochloride Structure in the drug label.
Pharmacodynamics
There is limited information regarding Idarubicin hydrochloride Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Idarubicin hydrochloride Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Idarubicin hydrochloride Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Idarubicin hydrochloride Clinical Studies in the drug label.
How Supplied
There is limited information regarding Idarubicin hydrochloride How Supplied in the drug label.
Storage
There is limited information regarding Idarubicin hydrochloride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Idarubicin hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Idarubicin hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Idarubicin hydrochloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Idarubicin hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Idarubicin hydrochloride Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Idarubicin hydrochloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.