Hydroxyzine (oral): Difference between revisions
Shanshan Cen (talk | contribs) No edit summary |
m (Protected "Hydroxyzine (oral)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (27 intermediate revisions by 3 users not shown) | |||
| Line 12: | Line 12: | ||
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested. | For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested. | ||
Useful in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus. | Useful in the management of [[pruritus]] due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus. | ||
As a sedative when used as premedication and following general anesthesia, Hydroxyzine may potentiate meperidine (Demerol®) and barbiturates, so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. Hydroxyzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent. | As a [[sedative]] when used as premedication and following general [[anesthesia]], Hydroxyzine may potentiate [[meperidine]] (Demerol®) and [[barbiturates]], so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. Hydroxyzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent. | ||
The effectiveness of hydroxyzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient. | The effectiveness of hydroxyzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient. | ||
| Line 28: | Line 28: | ||
As with all medications, the dosage should be adjusted according to the patient’s response to therapy. | As with all medications, the dosage should be adjusted according to the patient’s response to therapy. | ||
|offLabelAdultGuideSupport====== | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|fdaLIADPed=====Indications==== | |||
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested. | |||
Useful in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus. | |||
As a sedative when used as premedication and following general anesthesia, Hydroxyzine may potentiate meperidine (Demerol®) and barbiturates, so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. Hydroxyzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent. | |||
The effectiveness of hydroxyzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient. | |||
====Dosage==== | |||
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: children under 6 years, 50 mg daily in divided doses: children over 6 years: 50-100 mg daily in divided doses. | |||
For use in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus: children under 6 years, 50 mg daily in divided doses and children over 6 years, 50-100 mg daily in divided doses. | For use in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus: children under 6 years, 50 mg daily in divided doses and children over 6 years, 50-100 mg daily in divided doses. | ||
| Line 67: | Line 49: | ||
As with all medications, the dosage should be adjusted according to the patient’s response to therapy. | As with all medications, the dosage should be adjusted according to the patient’s response to therapy. | ||
|offLabelPedGuideSupport=== | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|contraindications=Oral hydroxyzine hydrochloride is contraindicated in patients with known hypersensitivity to hydroxyzine hydrochloride products, and in patients with known hypersensitivity to cetirizine hydrochloride or levocetirizine hydrochloride. | |||
Hydroxyzine, when administered to the pregnant mouse, rat and rabbit, induced fetal abnormalities in the rat and mouse at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy. | |||
* | Hydroxyzine is contraindicated for patients who have shown a previous hypersensitivity to it. | ||
|warnings=*Keep out of reach of children. | |||
* | *Nursing mothers: It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydroxyzine should not be given to nursing mothers. | ||
|clinicalTrials=There is limited information regarding ''Clinical Trial Experience'' of Hydroxyzine (oral) in adult patients. | |||
|postmarketing=====Skin and Appendages==== | |||
Oral hydroxyzine hydrochloride is associated with fixed drug eruptions in postmarketing reports. | |||
Side effects reported with the administration of hydroxyzine hydrochloride are usually mild and transitory in nature. | |||
====Anticholinergic==== | |||
[[Dry mouth]]. | |||
====Central Nervous System==== | |||
[[Drowsiness]] is usually transitory and may disappear in a few days of continued therapy or upon reduction of the dose. Involuntary motor activity including rare instances of [[tremor]] and [[Seizure|convulsions]] have been reported, usually with doses considerably higher than those recommended. Clinically significant [[Hypoventilation|respiratory depression]] has not been reported at recommended doses. | |||
|drugInteractions=There is limited information regarding ''Drug Interactions'' of Hydroxyzine (oral) in adult patients. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=Hydroxyzine, when administered to the pregnant mouse, rat and rabbit, induced fetal abnormalities in the rat and mouse at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy. | |||
|AUSPregCat=A | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydroxyzine should not be given to nursing mothers. | |||
|useInGeri=A determination has not been made whether controlled clinical studies of hydroxyzine hydrochloride included sufficient numbers of subjects aged 65 and over to define a difference in response from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. | |||
The extent of renal excretion of hydroxyzine hydrochloride has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections. | |||
Sedating drugs may cause confusion and over sedation in the elderly; elderly patients generally should be started on low doses of hydroxyzine hydrochloride and observed closely. | |||
|administration=* Oral | |administration=* Oral | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
|overdose=The most common manifestation of hydroxyzine hydrochloride overdosage is hypersedation. As in the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken. | |||
If [[vomiting]] has not occurred spontaneously, it should be induced. Immediate [[gastric lavage]] is also recommended. General supportive care, including frequent monitoring of the [[vital signs]] and close observation of the patient, is indicated. [[Hypotension]], though unlikely, may be controlled with intravenous fluids and Levophed® (levarterenol), or Aramine® (metaraminol). Do not use [[epinephrine]] as hydroxyzine hydrochloride counteracts its pressor action. | |||
There is no specific antidote. It is doubtful that [[hemodialysis]] would be of any value in the treatment of overdosage with hydroxyzine. However, if other agents such as barbiturates have been ingested concomitantly, [[hemodialysis]] may be indicated. There is no practical method to quantitate hydroxyzine in body fluids or tissue after its ingestion or administration. | |||
| | |drugBox={{Drugbox2 | ||
| verifiedrevid = 461774177 | |||
| IUPAC_name = (±)-2-(2-{4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl}ethoxy)ethanol | |||
| image = Hydroxyzine.png | |||
| image2 = Hydroxyzine-3d-sticks.png | |||
<!-- | <!--Clinical data--> | ||
| | | tradename = Vistaril | ||
| Drugs.com = {{drugs.com|monograph|hydroxyzine-hydrochloride}} | |||
| MedlinePlus = a682866 | |||
| pregnancy_AU = A | |||
| pregnancy_US = C | |||
| legal_AU = S4 | |||
| legal_status = Rx-only | |||
| routes_of_administration = [[Mouth|Oral]], [[Intramuscular injection|IM]] | |||
==== | <!--Pharmacokinetic data--> | ||
| bioavailability = High [[In vivo|in-vivo]] | |||
| protein_bound = 93% | |||
| metabolism = [[Liver|Hepatic]] | |||
| elimination_half-life = 20–24 hours<ref name="pmid6141198">{{cite journal | author = Simons FE, Simons KJ, Frith EM | title = The pharmacokinetics and antihistaminic of the H1 receptor antagonist hydroxyzine | journal = The Journal of Allergy and Clinical Immunology | volume = 73 | issue = 1 Pt 1 | pages = 69–75 |date=January 1984 | pmid = 6141198 | doi = 10.1016/0091-6749(84)90486-x| url = }}</ref> | |||
| excretion = [[Urine]], [[Feces]] | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 68-88-2 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = BB01 | |||
| PubChem = 3658 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00557 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3531 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 30S50YM8OG | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08054 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 5818 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 896 | |||
==== | <!--Chemical data--> | ||
| C = 21 | H = 27 | Cl = 1 | N = 2 | O = 2 | |||
| molecular_weight = 374.904 g/mol | |||
| smiles = Clc1ccc(cc1)C(c2ccccc2)N3CCN(CC3)CCOCCO | |||
| InChI = 1/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | |||
| InChIKey = ZQDWXGKKHFNSQK-UHFFFAOYAL | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = ZQDWXGKKHFNSQK-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction=Hydroxyzine hydrochloride is not a cortical [[depressant]], but its action may be due to a suppression of activity in certain key regions of the subcortical area of the [[central nervous system]]. Primary skeletal muscle relaxation has been demonstrated experimentally. Bronchodilator activity, and [[antihistaminic]] and [[analgesic]] effects have been demonstrated experimentally and confirmed clinically. An antiemetic effect, both by the [[apomorphine]] test and the veriloid test, has been demonstrated. Pharmacological and clinical studies indicate that hydroxyzine in therapeutic dosage does not increase gastric secretion or acidity and in most cases has mild antisecretory activity. | |||
|structure=Hydroxyzine hydrochloride is designated chemically as 1-(p-chlorobenzhydryl) 4-[2-(2- hydroxyethoxy)-ethyl] piperazine dihydrochloride, with the following structure: | |||
[[File:Hydroxyzine oral1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Each 5 mL (teaspoonful), for oral administration, contains 10 mg of hydroxyzine hydrochloride. | |||
The inactive ingredients for the oral solution are alcohol 0.5% v/v, corn syrup, methylparaben, peppermint flavor, propylene glycol, purified water, sodium benzoate, and sucrose. Sodium hydroxide and/or hydrochloric acid may be used to adjust the pH when necessary. | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
|PK=Hydroxyzine is rapidly absorbed from the gastrointestinal tract and its clinical effects are usually noted within 15 to 30 minutes after oral administration. | |||
|PK= | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | ||
|howSupplied=Hydroxyzine Hydrochloride Oral Solution, USP 10 mg per 5 mL (teaspoonful) is a slightly yellow peppermint flavored liquid supplied in 1 pint amber plastic bottles.Alcohol content: alcohol 0.5%v/v. | |||
|storage=Store at controlled room temperature 15° - 30°C (59° - 86°F). | |||
|howSupplied= | |packLabel=[[File:Hydroxyzine oral2.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
| | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* ®<ref>{{Cite web | title = | |brandNames=* HYDROXYZINE HYDROCHLORIDE ®<ref>{{Cite web | title =HYDROXYZINE HYDROCHLORIDE- hydroxyzine hydrochloride solution | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c848b1ae-2216-48f0-8646-165ecf7ed39b}}</ref> | ||
|lookAlike=* hydrOXYzine — hydrALAZINE <ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
* hydrOXYzine — Hydrogesic ®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
|lookAlike=* | |||
< | |||
{{ | |||
| | |||
| | |||
| | |||
}} | |||
}} | }} | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 16:29, 20 August 2015
{{DrugProjectFormSinglePage |authorTag=Shanshan Cen, M.D. [1] |genericName=Hydroxyzine hydrochloride |aOrAn=an |drugClass=antianxiety, antiemetic, central nervous system agent |indicationType=treatment |indication=anxiety and tension associated with psychoneurosis and pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses. It can be also used as a sedative when used as premedication and following general anesthesia |adverseReactions=xerostomia, headache, and somnolence. |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=====Indications==== For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested.
Useful in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus.
As a sedative when used as premedication and following general anesthesia, Hydroxyzine may potentiate meperidine (Demerol®) and barbiturates, so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. Hydroxyzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent.
The effectiveness of hydroxyzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient.
Dosage
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: in adults, 50-100 mg q.i.d.
For use in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus: in adults, 25 mg t.i.d. or q.i.d.
As a sedative when used as a premedication and following general anesthesia: 50-100 mg in adults.
When treatment is initiated by the intramuscular route of administration, subsequent doses may be administered orally.
As with all medications, the dosage should be adjusted according to the patient’s response to therapy. |offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Hydroxyzine (oral) in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroxyzine (oral) in adult patients. |fdaLIADPed=====Indications==== For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested.
Useful in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus.
As a sedative when used as premedication and following general anesthesia, Hydroxyzine may potentiate meperidine (Demerol®) and barbiturates, so their use in pre-anesthetic adjunctive therapy should be modified on an individual basis. Atropine and other belladonna alkaloids are not affected by the drug. Hydroxyzine is not known to interfere with the action of digitalis in any way and it may be used concurrently with this agent.
The effectiveness of hydroxyzine as an antianxiety agent for long term use, that is more than 4 months, has not been assessed by systematic clinical studies. The physician should reassess periodically the usefulness of the drug for the individual patient.
Dosage
For symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested: children under 6 years, 50 mg daily in divided doses: children over 6 years: 50-100 mg daily in divided doses.
For use in the management of pruritus due to allergic conditions such as chronic urticaria and atopic and contact dermatoses, and in histamine-mediated pruritus: children under 6 years, 50 mg daily in divided doses and children over 6 years, 50-100 mg daily in divided doses.
As a sedative when used as a premedication and following general anesthesia: 0.6 mg/kg in children.
When treatment is initiated by the intramuscular route of administration, subsequent doses may be administered orally.
As with all medications, the dosage should be adjusted according to the patient’s response to therapy. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Hydroxyzine (oral) in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Hydroxyzine (oral) in pediatric patients. |contraindications=Oral hydroxyzine hydrochloride is contraindicated in patients with known hypersensitivity to hydroxyzine hydrochloride products, and in patients with known hypersensitivity to cetirizine hydrochloride or levocetirizine hydrochloride.
Hydroxyzine, when administered to the pregnant mouse, rat and rabbit, induced fetal abnormalities in the rat and mouse at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy.
Hydroxyzine is contraindicated for patients who have shown a previous hypersensitivity to it. |warnings=*Keep out of reach of children.
- Nursing mothers: It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydroxyzine should not be given to nursing mothers.
|clinicalTrials=There is limited information regarding Clinical Trial Experience of Hydroxyzine (oral) in adult patients. |postmarketing=====Skin and Appendages==== Oral hydroxyzine hydrochloride is associated with fixed drug eruptions in postmarketing reports.
Side effects reported with the administration of hydroxyzine hydrochloride are usually mild and transitory in nature.
Anticholinergic
Central Nervous System
Drowsiness is usually transitory and may disappear in a few days of continued therapy or upon reduction of the dose. Involuntary motor activity including rare instances of tremor and convulsions have been reported, usually with doses considerably higher than those recommended. Clinically significant respiratory depression has not been reported at recommended doses. |drugInteractions=There is limited information regarding Drug Interactions of Hydroxyzine (oral) in adult patients. |FDAPregCat=C |useInPregnancyFDA=Hydroxyzine, when administered to the pregnant mouse, rat and rabbit, induced fetal abnormalities in the rat and mouse at doses substantially above the human therapeutic range. Clinical data in human beings are inadequate to establish safety in early pregnancy. Until such data are available, hydroxyzine is contraindicated in early pregnancy. |AUSPregCat=A |useInLaborDelivery=There is no FDA guidance on use of Hydroxyzine (oral) during labor and delivery. |useInNursing=It is not known whether this drug is excreted in human milk. Since many drugs are so excreted, hydroxyzine should not be given to nursing mothers. |useInGeri=A determination has not been made whether controlled clinical studies of hydroxyzine hydrochloride included sufficient numbers of subjects aged 65 and over to define a difference in response from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
The extent of renal excretion of hydroxyzine hydrochloride has not been determined. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selections.
Sedating drugs may cause confusion and over sedation in the elderly; elderly patients generally should be started on low doses of hydroxyzine hydrochloride and observed closely. |administration=* Oral |monitoring=There is limited information regarding Monitoring of Hydroxyzine (oral) in the drug label. |overdose=The most common manifestation of hydroxyzine hydrochloride overdosage is hypersedation. As in the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken.
If vomiting has not occurred spontaneously, it should be induced. Immediate gastric lavage is also recommended. General supportive care, including frequent monitoring of the vital signs and close observation of the patient, is indicated. Hypotension, though unlikely, may be controlled with intravenous fluids and Levophed® (levarterenol), or Aramine® (metaraminol). Do not use epinephrine as hydroxyzine hydrochloride counteracts its pressor action.
There is no specific antidote. It is doubtful that hemodialysis would be of any value in the treatment of overdosage with hydroxyzine. However, if other agents such as barbiturates have been ingested concomitantly, hemodialysis may be indicated. There is no practical method to quantitate hydroxyzine in body fluids or tissue after its ingestion or administration. |drugBox={{Drugbox2 | verifiedrevid = 461774177 | IUPAC_name = (±)-2-(2-{4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl}ethoxy)ethanol | image = Hydroxyzine.png | image2 = Hydroxyzine-3d-sticks.png
| tradename = Vistaril | Drugs.com = Monograph | MedlinePlus = a682866 | pregnancy_AU = A | pregnancy_US = C | legal_AU = S4 | legal_status = Rx-only | routes_of_administration = Oral, IM
| bioavailability = High in-vivo | protein_bound = 93% | metabolism = Hepatic | elimination_half-life = 20–24 hours[1] | excretion = Urine, Feces
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 68-88-2
| ATC_prefix = N05
| ATC_suffix = BB01
| PubChem = 3658
| DrugBank_Ref =
| DrugBank = DB00557
| ChemSpiderID_Ref =
| ChemSpiderID = 3531
| UNII_Ref =
| UNII = 30S50YM8OG
| KEGG_Ref =
| KEGG = D08054
| ChEBI_Ref =
| ChEBI = 5818
| ChEMBL_Ref =
| ChEMBL = 896
| C = 21 | H = 27 | Cl = 1 | N = 2 | O = 2
| molecular_weight = 374.904 g/mol
| smiles = Clc1ccc(cc1)C(c2ccccc2)N3CCN(CC3)CCOCCO
| InChI = 1/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2
| InChIKey = ZQDWXGKKHFNSQK-UHFFFAOYAL
| StdInChI_Ref =
| StdInChI = 1S/C21H27ClN2O2/c22-20-8-6-19(7-9-20)21(18-4-2-1-3-5-18)24-12-10-23(11-13-24)14-16-26-17-15-25/h1-9,21,25H,10-17H2
| StdInChIKey_Ref =
| StdInChIKey = ZQDWXGKKHFNSQK-UHFFFAOYSA-N
}}
|mechAction=Hydroxyzine hydrochloride is not a cortical depressant, but its action may be due to a suppression of activity in certain key regions of the subcortical area of the central nervous system. Primary skeletal muscle relaxation has been demonstrated experimentally. Bronchodilator activity, and antihistaminic and analgesic effects have been demonstrated experimentally and confirmed clinically. An antiemetic effect, both by the apomorphine test and the veriloid test, has been demonstrated. Pharmacological and clinical studies indicate that hydroxyzine in therapeutic dosage does not increase gastric secretion or acidity and in most cases has mild antisecretory activity. |structure=Hydroxyzine hydrochloride is designated chemically as 1-(p-chlorobenzhydryl) 4-[2-(2- hydroxyethoxy)-ethyl] piperazine dihydrochloride, with the following structure:
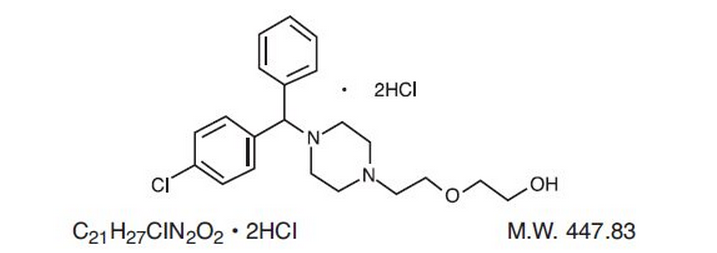
Each 5 mL (teaspoonful), for oral administration, contains 10 mg of hydroxyzine hydrochloride.
The inactive ingredients for the oral solution are alcohol 0.5% v/v, corn syrup, methylparaben, peppermint flavor, propylene glycol, purified water, sodium benzoate, and sucrose. Sodium hydroxide and/or hydrochloric acid may be used to adjust the pH when necessary. |PD=There is limited information regarding Pharmacodynamics of Hydroxyzine (oral) in the drug label. |PK=Hydroxyzine is rapidly absorbed from the gastrointestinal tract and its clinical effects are usually noted within 15 to 30 minutes after oral administration. |nonClinToxic=There is limited information regarding Nonclinical Toxicology of Hydroxyzine (oral) in the drug label. |clinicalStudies=There is limited information regarding Clinical Studies of Hydroxyzine (oral) in the drug label. |howSupplied=Hydroxyzine Hydrochloride Oral Solution, USP 10 mg per 5 mL (teaspoonful) is a slightly yellow peppermint flavored liquid supplied in 1 pint amber plastic bottles.Alcohol content: alcohol 0.5%v/v. |storage=Store at controlled room temperature 15° - 30°C (59° - 86°F).
|packLabel=

|fdaPatientInfo=There is limited information regarding Patient Counseling Information of Hydroxyzine (oral) in the drug label. |alcohol=* Alcohol-Hydroxyzine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=* HYDROXYZINE HYDROCHLORIDE ®[2] |lookAlike=* hydrOXYzine — hydrALAZINE [3]
- hydrOXYzine — Hydrogesic ®[3]
}}
- ↑ Simons FE, Simons KJ, Frith EM (January 1984). "The pharmacokinetics and antihistaminic of the H1 receptor antagonist hydroxyzine". The Journal of Allergy and Clinical Immunology. 73 (1 Pt 1): 69–75. doi:10.1016/0091-6749(84)90486-x. PMID 6141198.
- ↑ "HYDROXYZINE HYDROCHLORIDE- hydroxyzine hydrochloride solution".
- ↑ 3.0 3.1 "http://www.ismp.org". External link in
|title=(help)