Glycosaminoglycan
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
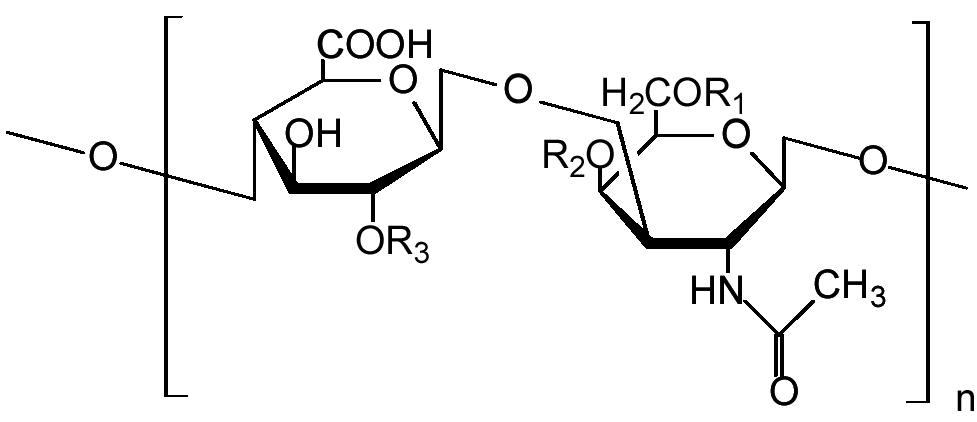

Glycosaminoglycans (GAGs) or mucopolysaccharides are long unbranched polysaccharides consisting of a repeating disaccharide unit.
Production
Protein cores made in the rough endoplasmic reticulum are posttranslationally modified by glycosyltransferases in the Golgi apparatus, where GAG disaccharides are added to protein cores; the exception is hyaluronan, which is uniquely synthesized without a protein core and is "spun out" by enzymes at cell surfaces directly into the extracellular space.
Structure
This unit consists of an N-acetyl-hexosamine and a hexose or hexuronic acid, either or both of which may be sulfated.
The combination of the sulfate group and the carboxylate groups of the uronic acid residues gives them a very high density of negative charge.
Function
This family of carbohydrates is essential or important for the life of vertebrates and an assortment of lower animals.
GAGs form an important component of connective tissues. GAG chains may be covalently linked to a protein to form proteoglycans.
Some examples of glycoaminoglycan uses in nature include heparin as an anticoagulant, hyaluronate as a component in the synovial fluid lubricant in body joints, and chondroitins which can be found in connective tissues, cartilage and tendons.
Classification
Members of the glycosaminoglycan family vary in the type of hexosamine, hexose or hexuronic acid unit they contain (e.g. glucuronic acid, iduronic acid, galactose, galactosamine, glucosamine).
They also vary in the geometry of the glycosidic linkage.
Examples of GAGs include:
| Name | Hexuronic acid / Hexose | Hexosamine | Linkage geometry between predominant monomeric units | Unique features |
| Chondroitin sulphate | GlcUA or GlcUA(2S) | GalNAc or GalNAc(4S) or GalNAc(6S) or GalNAc(4S,6S) | -4GlcUAβ1-3GalNAcβ1- | Most prevalent GAG |
| Dermatan sulphate | GlcUA or IdoUA or IdoUA(2S) | GalNAc or GalNAc(4S) or GalNAc(6S) or GalNAc(4S,6S) | -4IdoUAβ1-3GalNAcβ1- | Distinguished from chondroitin sulfate by the presence of iduronic acid, although some hexuronic acid monosaccharides may be glucuronic acid.[1] |
| Keratan sulphate | Gal or Gal(6S) | GlcNAc or GlcNAc(6S) | -3Gal(6S)β1-4GlcNAc(6S)β1- | Keratan sulfate type II may be fucosylated.[2] |
| Heparin | GlcUA or IdoUA(2S) | GlcNAc or GlcNS or GlcNAc(6S) or GlcNS(6S) | -4IdoUA(2S)α1-4GlcNS(6S)α1- | Highest negative charge density of any known biological molecule |
| Heparan sulfate | GlcUA or IdoUA or IdoUA(2S) | GlcNAc or GlcNS or GlcNAc(6S) or GlcNS(6S) | -4GlcUAβ1-4GlcNAcα1- | Highly similar in structure to heparin, however heparan sulfates disaccharide units are organised into distinct sulfated and non-sulfated domains.[3] |
| Hyaluronan | GlcUA | GlcNAc | -4GlcUAβ1-3GlcNAcβ1- | The only GAG that is exclusively non-sulfated |
Abbreviations
- GlcUA = β-D-glucuronic acid
- GlcUA(2S) = 2-O-sulfo-β-D-glucuronic acid
- IdoUA = α-L-iduronic acid
- IdoUA(2S) = 2-O-sulfo-α-L-iduronic acid
- Gal = β-D-galactose
- Gal(6S) = 6-O-sulfo-β-D-galactose
- GalNAc = β-D-N-acetylgalactosamine
- GalNAc(4S) = β-D-N-acetylgalactosamine-4-O-sulfate
- GalNAc(6S) = β-D-N-acetylgalactosamine-6-O-sulfate
- GalNAc(4S,6S) = β-D-N-acetylgalactosamine-4-O, 6-O-sulfate
- GlcNAc = α-D-N-acetylglucosamine
- GlcNS = α-D-N-sulfoglucosamine
- GlcNS(6S) = α-D-N-sulfoglucosamine-6-O-sulfate
References
- ↑ Trowbridge JM, Gallo RL. (2002). "Dermatan sulfate: new functions from an old glycosaminoglycan". Glycobiology. 12 (9): 117R–125R. PMID 12213784.
- ↑ Funderburgh JL. (2000). "Keratan sulfate: structure, biosynthesis, and function". Glycobiology. 10 (10): 951–958. PMID 11030741.
- ↑ Gallagher, J.T., Lyon, M. (2000). "Molecular structure of Heparan Sulfate and interactions with growth factors and morphogens". In Iozzo, M, V. Proteoglycans: structure, biology and molecular interactions. Marcel Dekker Inc. New York, New York. pp. 27–59.
See also
- Mucopolysaccharidosis (lysosomal storage diseases)
- Lipopolysaccharide
External links
- Proteoglycans and Glycosaminoglycans at Lancaster University
- Illustration at scientificpsychic.com
- King M. 2005. Glycosaminoglycans. Indiana University School of Medicine Accessed December 31, 2006.
- Glycosaminoglycans at the US National Library of Medicine Medical Subject Headings (MeSH)
de:Glykosaminoglykane el:Γλυκοζαμινογλυκάνες it:Glicosaminoglicano nl:Glycosaminoglycaan sk:Glykosaminoglykán