Glucose-6-phosphate dehydrogenase deficiency pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 36: | Line 36: | ||
===Pathogenesis=== | ===Pathogenesis=== | ||
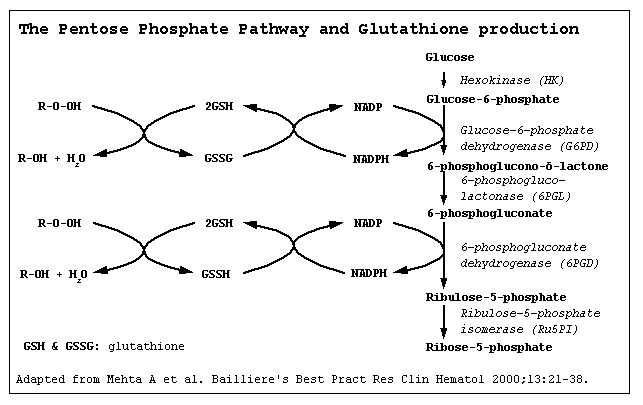
*It is understood that G6PD deficiency is the result of reduced Glucose-6-phosphate dehydrogenase enzyme levels. G6PD deficiency is an X-linked disorder. It is the most common enzymatic disorder of red blood cells. Glucose-6-phosphate dehydrogenase enzyme oxidize glucose-6-phosphate to 6-phosphogluconolactone in pentose phosphate pathway ( HMP shunt). Glucose-6-phosphate dehydrogenase enzyme also reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH. NADPH is an important cofactor in glutathione metabolism against oxidative injury in RBC.Reduced glutathione (GSH) convert to oxidized glutathione (GSSG) by glutathione peroxidase enzyme that prevent oxidant accumulation. Glutathione reductase catalyzes the reduction of GSSG to GSH by NADPH. In G6PD deficiency, oxidative stresses can denature hemoglobin and intravascular hemolysis in RBC can happen. Infection, some meication and foods with high level of convicine, vicine, divicine and isouramil such as fava beans can cause oxidative stress. Spleen is the organ for sequesteration damaged RBC. The hemoglobin is metabolized to bilirubin and cause jaundice. | *It is understood that G6PD deficiency is the result of reduced Glucose-6-phosphate dehydrogenase enzyme levels. G6PD deficiency is an X-linked disorder. It is the most common enzymatic disorder of red blood cells. Glucose-6-phosphate dehydrogenase enzyme oxidize glucose-6-phosphate to 6-phosphogluconolactone in pentose phosphate pathway ( HMP shunt). Glucose-6-phosphate dehydrogenase enzyme also reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH. NADPH is an important cofactor in glutathione metabolism against oxidative injury in RBC.Reduced glutathione (GSH) convert to oxidized glutathione (GSSG) by glutathione peroxidase enzyme that prevent oxidant accumulation. Glutathione reductase catalyzes the reduction of GSSG to GSH by NADPH. In G6PD deficiency, oxidative stresses can denature hemoglobin and intravascular hemolysis in RBC can happen. Infection, some meication and foods with high level of convicine, vicine, divicine and isouramil such as fava beans can cause oxidative stress. Spleen is the organ for sequesteration damaged RBC. The hemoglobin is metabolized to bilirubin and cause jaundice. | ||
*[Pathogen name] is usually transmitted via the [transmission route] route to the human host. | *[Pathogen name] is usually transmitted via the [transmission route] route to the human host. | ||
| Line 49: | Line 46: | ||
G6PD deficiency is transmitted in x-linked disorder pattern. The gene G6PD is located in the distal long arm of the X chromosome at the Xq28 locus. <ref name="pmid14033020">{{cite journal |vauthors=KIRKMAN HN, HENDRICKSON EM |title=Sex-linked electrophoretic difference in glucose-6-phosphate dehydrogenase |journal=Am. J. Hum. Genet. |volume=15 |issue= |pages=241–58 |date=September 1963 |pmid=14033020 |pmc=1932381 |doi= |url=}}</ref> | G6PD deficiency is transmitted in x-linked disorder pattern. The gene G6PD is located in the distal long arm of the X chromosome at the Xq28 locus. <ref name="pmid14033020">{{cite journal |vauthors=KIRKMAN HN, HENDRICKSON EM |title=Sex-linked electrophoretic difference in glucose-6-phosphate dehydrogenase |journal=Am. J. Hum. Genet. |volume=15 |issue= |pages=241–58 |date=September 1963 |pmid=14033020 |pmc=1932381 |doi= |url=}}</ref> | ||
Heterozygous women are usually normal because of lyonization ( X innactivation)<ref name="pmid13868717">{{cite journal |vauthors=BEUTLER E, YEH M, FAIRBANKS VF |title=The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=48 |issue= |pages=9–16 |date=January 1962 |pmid=13868717 |pmc=285481 |doi= |url=}}</ref> | |||
G6PD B, is the wild type or normal. G6PD has 400 variant enzymes. <ref name="pmid7949118">{{cite journal |vauthors=Beutler E |title=G6PD deficiency |journal=Blood |volume=84 |issue=11 |pages=3613–36 |date=December 1994 |pmid=7949118 |doi= |url=}}</ref> | |||
'''G6PD and its variants''' — The monomeric form of G6PD contains 515 amino acids, but the active form of G6PD is a dimer that contains tightly bound NADP [15,16]. Amino acid 205 is the binding site for glucose-6-phosphate, while amino acids 386 and 387 may be involved in binding to NADP [15,17]. | '''G6PD and its variants''' — The monomeric form of G6PD contains 515 amino acids, but the active form of G6PD is a dimer that contains tightly bound NADP [15,16]. Amino acid 205 is the binding site for glucose-6-phosphate, while amino acids 386 and 387 may be involved in binding to NADP [15,17]. | ||
The normal or wild-type enzyme is called G6PD B, although over 400 variant enzymes have been identified [15,16,18,19]. By international agreement, standardized methods have been used to characterize these enzyme variants, which differ on the basis of their biochemical properties, such as kinetic activity and the Michaelis constant for its substrate glucose-6-phosphate and cofactor NADP [20,21]. However, differences between some variants are subtle and may not represent true enzyme differences. | The normal or wild-type enzyme is called G6PD B,, although over 400 variant enzymes have been identified [15,16,18,19]. By international agreement, standardized methods have been used to characterize these enzyme variants, which differ on the basis of their biochemical properties, such as kinetic activity and the Michaelis constant for its substrate glucose-6-phosphate and cofactor NADP [20,21]. However, differences between some variants are subtle and may not represent true enzyme differences. | ||
The variants are almost all missense point mutations, although a few deletions have been described [15,18]. Large deletions or frame shift mutations have not been identified, suggesting that complete absence of G6PD may be lethal [15]. Most class I variants that are associated with chronic hemolytic anemia have abnormalities in the glucose-6-phosphate binding or NADP binding site of the enzyme (figure 2) [15]. | The variants are almost all missense point mutations, although a few deletions have been described [15,18]. Large deletions or frame shift mutations have not been identified, suggesting that complete absence of G6PD may be lethal [15]. Most class I variants that are associated with chronic hemolytic anemia have abnormalities in the glucose-6-phosphate binding or NADP binding site of the enzyme (figure 2) [15]. | ||
| Line 70: | Line 65: | ||
OR | OR | ||
The development of | The development of G6PD deficency is the result of missense point mutations and also a few deletions. <ref name="pmid2190319">{{cite journal |vauthors=Beutler E |title=The genetics of glucose-6-phosphate dehydrogenase deficiency |journal=Semin. Hematol. |volume=27 |issue=2 |pages=137–64 |date=April 1990 |pmid=2190319 |doi= |url=}}</ref>such as: | ||
*[Mutation 1] | *[Mutation 1] | ||
Revision as of 14:24, 14 August 2018
|
Glucose-6-phosphate dehydrogenase deficiency Microchapters |
|
Differentiating Glucose-6-phosphate dehydrogenase deficiency from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
FDA on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
CDC on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology in the news |
|
Blogs on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Directions to Hospitals Treating Glucose-6-phosphate dehydrogenase deficiency |
|
Risk calculators and risk factors for Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mahda Alihashemi M.D. [2]
Overview
The exact pathogenesis of [disease name] is not fully understood.
OR
It is thought that [disease name] is the result of / is mediated by / is produced by / is caused by either [hypothesis 1], [hypothesis 2], or [hypothesis 3].
OR
[Pathogen name] is usually transmitted via the [transmission route] route to the human host.
OR
Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
OR
[Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
OR
The progression to [disease name] usually involves the [molecular pathway].
OR
The pathophysiology of [disease/malignancy] depends on the histological subtype.
Pathophysiology
Physiology
The normal physiology of [name of process] can be understood as follows:
Pathogenesis
- It is understood that G6PD deficiency is the result of reduced Glucose-6-phosphate dehydrogenase enzyme levels. G6PD deficiency is an X-linked disorder. It is the most common enzymatic disorder of red blood cells. Glucose-6-phosphate dehydrogenase enzyme oxidize glucose-6-phosphate to 6-phosphogluconolactone in pentose phosphate pathway ( HMP shunt). Glucose-6-phosphate dehydrogenase enzyme also reduces nicotinamide adenine dinucleotide phosphate (NADP) to NADPH. NADPH is an important cofactor in glutathione metabolism against oxidative injury in RBC.Reduced glutathione (GSH) convert to oxidized glutathione (GSSG) by glutathione peroxidase enzyme that prevent oxidant accumulation. Glutathione reductase catalyzes the reduction of GSSG to GSH by NADPH. In G6PD deficiency, oxidative stresses can denature hemoglobin and intravascular hemolysis in RBC can happen. Infection, some meication and foods with high level of convicine, vicine, divicine and isouramil such as fava beans can cause oxidative stress. Spleen is the organ for sequesteration damaged RBC. The hemoglobin is metabolized to bilirubin and cause jaundice.
- [Pathogen name] is usually transmitted via the [transmission route] route to the human host.
- Following transmission/ingestion, the [pathogen] uses the [entry site] to invade the [cell name] cell.
- [Disease or malignancy name] arises from [cell name]s, which are [cell type] cells that are normally involved in [function of cells].
- The progression to [disease name] usually involves the [molecular pathway].
- The pathophysiology of [disease/malignancy] depends on the histological subtype.
Genetics
G6PD deficiency is transmitted in x-linked disorder pattern. The gene G6PD is located in the distal long arm of the X chromosome at the Xq28 locus. [1]
Heterozygous women are usually normal because of lyonization ( X innactivation)[2]
G6PD B, is the wild type or normal. G6PD has 400 variant enzymes. [3]
G6PD and its variants — The monomeric form of G6PD contains 515 amino acids, but the active form of G6PD is a dimer that contains tightly bound NADP [15,16]. Amino acid 205 is the binding site for glucose-6-phosphate, while amino acids 386 and 387 may be involved in binding to NADP [15,17].
The normal or wild-type enzyme is called G6PD B,, although over 400 variant enzymes have been identified [15,16,18,19]. By international agreement, standardized methods have been used to characterize these enzyme variants, which differ on the basis of their biochemical properties, such as kinetic activity and the Michaelis constant for its substrate glucose-6-phosphate and cofactor NADP [20,21]. However, differences between some variants are subtle and may not represent true enzyme differences.
The variants are almost all missense point mutations, although a few deletions have been described [15,18]. Large deletions or frame shift mutations have not been identified, suggesting that complete absence of G6PD may be lethal [15]. Most class I variants that are associated with chronic hemolytic anemia have abnormalities in the glucose-6-phosphate binding or NADP binding site of the enzyme (figure 2) [15].
OR
Genes involved in the pathogenesis of G6PD deficency include:
- [Gene1]
- [Gene2]
- [Gene3]
OR
The development of G6PD deficency is the result of missense point mutations and also a few deletions. [4]such as:
- [Mutation 1]
- [Mutation 2]
- [Mutation 3]
Associated Conditions
Gross Pathology
On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
Microscopic Pathology
On microscopic histopathological analysis, , Heinz bodies can be visualized as a result of denatured hemoglobin in peripheral blood smears with supravital staining. processed with supravital staining (Heinz body prep).
References
- ↑ KIRKMAN HN, HENDRICKSON EM (September 1963). "Sex-linked electrophoretic difference in glucose-6-phosphate dehydrogenase". Am. J. Hum. Genet. 15: 241–58. PMC 1932381. PMID 14033020.
- ↑ BEUTLER E, YEH M, FAIRBANKS VF (January 1962). "The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker". Proc. Natl. Acad. Sci. U.S.A. 48: 9–16. PMC 285481. PMID 13868717.
- ↑ Beutler E (December 1994). "G6PD deficiency". Blood. 84 (11): 3613–36. PMID 7949118.
- ↑ Beutler E (April 1990). "The genetics of glucose-6-phosphate dehydrogenase deficiency". Semin. Hematol. 27 (2): 137–64. PMID 2190319.
|
Glucose-6-phosphate dehydrogenase deficiency Microchapters |
|
Differentiating Glucose-6-phosphate dehydrogenase deficiency from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology On the Web |
|
American Roentgen Ray Society Images of Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
FDA on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
CDC on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Glucose-6-phosphate dehydrogenase deficiency pathophysiology in the news |
|
Blogs on Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
|
Directions to Hospitals Treating Glucose-6-phosphate dehydrogenase deficiency |
|
Risk calculators and risk factors for Glucose-6-phosphate dehydrogenase deficiency pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]; Associate Editor(s)-In-Chief: Priyamvada Singh, M.D. [4]
Please help WikiDoc by adding content here. It's easy! Click here to learn about editing.
Overview
Pathophysiology