Fondaparinux: Difference between revisions
No edit summary |
No edit summary |
||
| Line 189: | Line 189: | ||
[[image:Fondaparinux Table 2|thumb|left|600px| | [[image:Fondaparinux Table 2|thumb|left|600px| | ||
<sup>a</sup> [[Enoxaparin]] sodium dosing regimen: 30 mg every 12 hours or 40 mg once daily. | <sup>a</sup> [[Enoxaparin]] sodium dosing regimen: 30 mg every 12 hours or 40 mg once daily.<br> <sup>b</sup> Not approved for use in patients undergoing hip fracture surgery.<br> <sup>c</sup> Major bleeding was defined as clinically overt bleeding that was (1) fatal, (2) bleeding at critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), (3) associated with re-operation at operative site, or (4) with a bleeding index (BI) ≥2. <br> <sup>d</sup> BI ≥2: Overt bleeding associated only with a bleeding index (BI) ≥2 calculated as [number of whole blood or packed red blood cell units transfused + [(pre-bleeding) – (post-bleeding)] [[hemoglobin ]](g/dL) values]. <br><sup>e</sup> Minor bleeding was defined as clinically overt bleeding that was not major.]] | ||
<sup>b</sup> Not approved for use in patients undergoing hip fracture surgery. | |||
<sup>c</sup> Major bleeding was defined as clinically overt bleeding that was (1) fatal, (2) bleeding at critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), (3) associated with re-operation at operative site, or (4) with a bleeding index (BI) ≥2. | |||
<sup>d</sup> BI ≥2: Overt bleeding associated only with a bleeding index (BI) ≥2 calculated as [number of whole blood or packed red blood cell units transfused + [(pre-bleeding) – (post-bleeding)] [[hemoglobin ]](g/dL) values]. | |||
<sup>e</sup> Minor bleeding was defined as clinically overt bleeding that was not major.]] | |||
{{clr}} | {{clr}} | ||
A separate analysis of major bleeding across all randomized, controlled, peri-operative, prophylaxis clinical studies of hip fracture, hip replacement, or knee replacement surgery according to the time of the first injection of fondaparinux after surgical closure was performed in patients who received fondaparinux only post-operatively. In this analysis, the incidences of major bleeding were as follows: <4 hours was 4.8% (5/104), 4 to 6 hours was 2.3% (28/1,196), 6 to 8 hours was 1.9% (38/1,965). In all studies, the majority (≥75%) of the major bleeding events occurred during the first 4 days after surgery. | A separate analysis of major bleeding across all randomized, controlled, peri-operative, prophylaxis clinical studies of hip fracture, hip replacement, or knee replacement surgery according to the time of the first injection of fondaparinux after surgical closure was performed in patients who received fondaparinux only post-operatively. In this analysis, the incidences of major bleeding were as follows: <4 hours was 4.8% (5/104), 4 to 6 hours was 2.3% (28/1,196), 6 to 8 hours was 1.9% (38/1,965). In all studies, the majority (≥75%) of the major bleeding events occurred during the first 4 days after surgery. | ||
| Line 200: | Line 196: | ||
[[image:Fondaparinux Table 3|thumb|left|600px| | [[image:Fondaparinux Table 3|thumb|left|600px| | ||
<sup>a</sup> Major bleeding was defined as bleeding that was (1) fatal, (2) bleeding at the surgical site leading to intervention, (3) non-surgical bleeding at a critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), or leading to an intervention, and/or with a bleeding index (BI) ≥2. | <sup>a</sup> Major bleeding was defined as bleeding that was (1) fatal, (2) bleeding at the surgical site leading to intervention, (3) non-surgical bleeding at a critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), or leading to an intervention, and/or with a bleeding index (BI) ≥2.<br> <sup>b</sup> Minor bleeding was defined as clinically overt bleeding that was not major.]] | ||
<sup>b</sup> Minor bleeding was defined as clinically overt bleeding that was not major.]] | |||
{{clr}} | {{clr}} | ||
The rates of major bleeding according to the time interval following the first fondaparinux injection were as follows: <6 hours was 3.4% (9/263) and 6 to 8 hours was 2.9% (32/1112). | The rates of major bleeding according to the time interval following the first fondaparinux injection were as follows: <6 hours was 3.4% (9/263) and 6 to 8 hours was 2.9% (32/1112). | ||
| Line 208: | Line 203: | ||
[[image:Fondaparinux Table 4|thumb|left|600px| | [[image:Fondaparinux Table 4|thumb|left|600px| | ||
<sup>a</sup> Bleeding rates are during the study drug treatment period (approximately 7 days). Patients were also treated with[[ vitamin K antagonists]] initiated within 72 hours after the first study drug administration. | <sup>a</sup> Bleeding rates are during the study drug treatment period (approximately 7 days). Patients were also treated with[[ vitamin K antagonists]] initiated within 72 hours after the first study drug administration.<br> <sup>b</sup> Major bleeding was defined as clinically overt: –and/or contributing to death – and/or in a critical organ including intracranial, retroperitoneal, intraocular, spinal, pericardial, or adrenal gland – and/or associated with a fall in hemoglobin level ≥2 g/dL – and/or leading to a transfusion ≥2 units of packed red blood cells or whole blood.<br><sup>c</sup> Clinically overt bleeding with a 2 g/dL fall in hemoglobin and/or leading to transfusion of PRBC or whole blood ≥2 units.<br> | ||
<sup>b</sup> Major bleeding was defined as clinically overt: –and/or contributing to death – and/or in a critical organ including intracranial, retroperitoneal, intraocular, spinal, pericardial, or adrenal gland – and/or associated with a fall in hemoglobin level ≥2 g/dL – and/or leading to a transfusion ≥2 units of packed red blood cells or whole blood. | |||
<sup>c</sup> Clinically overt bleeding with a 2 g/dL fall in hemoglobin and/or leading to transfusion of PRBC or whole blood ≥2 units. | |||
<sup>d</sup> Minor bleeding was defined as clinically overt bleeding that was not major.]] | <sup>d</sup> Minor bleeding was defined as clinically overt bleeding that was not major.]] | ||
{{clr}} | {{clr}} | ||
| Line 229: | Line 222: | ||
Other adverse reactions that occurred during treatment with fondaparinux in clinical trials with patients undergoing hip fracture, hip replacement, or knee replacement surgery are provided in Table 5. | Other adverse reactions that occurred during treatment with fondaparinux in clinical trials with patients undergoing hip fracture, hip replacement, or knee replacement surgery are provided in Table 5. | ||
[[image:Fondaparinux Table 5|thumb|left|600px|]] | [[image:Fondaparinux Table 5|thumb|left|600px| <sup>a</sup> Enoxaparin sodium dosing regimen: 30 mg every 12 hours or 40 mg once daily.<br> <sup>b</sup> Not approved for use in patients undergoing hip fracture surgery. <br> <sup>c</sup> Localized blister coded as bullous eruption.]] | ||
{{clr}} | {{clr}} | ||
Adverse reactions in the abdominal surgery study and in the [[VTE]] treatment trials generally occurred at lower rates than in the hip and knee surgery trials described above. The most common adverse reaction in the abdominal surgery trial was post-operative wound infection (4.9%), and the most common adverse reaction in the [[VTE]] treatment trials was [[epistaxis]](1.3%). | Adverse reactions in the abdominal surgery study and in the [[VTE]] treatment trials generally occurred at lower rates than in the hip and knee surgery trials described above. The most common adverse reaction in the abdominal surgery trial was post-operative wound infection (4.9%), and the most common adverse reaction in the [[VTE]] treatment trials was [[epistaxis]](1.3%). | ||
|postmarketing=The following adverse reactions have been identified during post-approval use of fondaparinux. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |postmarketing=The following adverse reactions have been identified during post-approval use of fondaparinux. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
Revision as of 14:24, 7 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SPINAL/EPIDURAL HEMATOMAS
See full prescribing information for complete Boxed Warning.
Spinal/Epidural Hematomas: Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH), heparinoids, or fondaparinux sodium and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurologic impairment. If neurologic compromise is noted, urgent treatment is necessary. Consider the benefit and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Overview
Fondaparinux is a actor Xa inhibitor that is FDA approved for the {{{indicationType}}} of prophylaxis of deep vein thrombosis (DVT) and DVT or acute pulmonary embolism (PE) when administered in conjunction with warfarin.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bleeding complications and thrombocytopenia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Deep Vein Thrombosis Prophylaxis
- Recommended dose: 2.5 mgadministered by subcutaneous injection once daily after hemostasis has been established.
- Administer the initial dose no earlier than 6 to 8 hours after surgery.
- Administration of Fondaparinux earlier than 6 hours after surgery increases the risk of major bleeding.
- The usual duration of therapy is 5 to 9 days; up to 11 days of therapy was administered in clinical trials.
- In patients undergoing hip fracture surgery, an extended prophylaxis course of up to 24 additional days is recommended.
- In patients undergoing hip fracture surgery, a total of 32 days (peri-operative and extended prophylaxis) was administered in clinical trials.
Deep Vein Thrombosis Prophylaxis Following Abdominal Surgery
- Recommended dose: 2.5 mg administered by subcutaneous injection once daily after hemostasis has been established.
- Administer the initial dose no earlier than 6 to 8 hours after surgery. Administration of fondaparinux earlier than 6 hours after surgery increases the risk of major bleeding.
- The usual duration of administration is 5 to 9 days, and up to 10 days of Fondaparinux was administered in clinical trials.
Deep Vein Thrombosis and Pulmonary Embolism Treatment
- Recommended dose: 5 mg (body weight <50 kg), 7.5 mg (body weight 50 to 100 kg), or 10 mg (body weight >100 kg) by subcutaneous injection once daily
- Initiate concomitant treatment with warfarin sodium as soon as possible, usually within 72 hours.
- Continue treatment with Fondaparinux for at least 5 days and until a therapeutic oral anticoagulant effect is established (INR 2 to 3).
- The usual duration of administration of Fondaparinux is 5 to 9 days; up to 26 days of Fondaparinux injection was administered in clinical trials.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Severe renal impairment (creatinine clearance (CrCl) < 30 mL/min).
- Active major bleeding.
- Bacterial endocarditis.
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of fondaparinux sodium.
- Body weight < 50 kg ( venous thromboembolism VTE prophylaxis only).
- History of serious hypersensitivity reaction (e.g., angioedema, anaphylactoid/anaphylactic reactions) to fondaparinux.
Warnings
|
WARNING: SPINAL/EPIDURAL HEMATOMAS
See full prescribing information for complete Boxed Warning.
Spinal/Epidural Hematomas: Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH), heparinoids, or fondaparinux sodium and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurologic impairment. If neurologic compromise is noted, urgent treatment is necessary. Consider the benefit and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis |
Hemorrhage
Use fondaparinux with extreme caution in conditions with increased risk of hemorrhage, such as congenital or acquired bleeding disorders, active ulcerative and angiodysplastic gastrointestinal disease, hemorrhagic stroke, uncontrolled arterial hypertension, diabetic retinopathy, or shortly after brain, spinal, or ophthalmological surgery. Isolated cases of elevated aPTT temporally associated with bleeding events have been reported following administration of fondaparinux (with or without concomitant administration of other anticoagulants).
Do not administer agents that enhance the risk of hemorrhage with fondaparinux unless essential for the management of the underlying condition, such as vitamin K antagonists for the treatment of VTE. If co-administration is essential, closely monitor patients for signs and symptoms of bleeding.
Do not administer the initial dose of fondaparinux earlier than 6 to 8 hours after surgery. Administration earlier than 6 hours after surgery increases risk of major bleeding.
Renal Impairment and Bleeding Risk
Fondaparinux increases the risk of bleeding in patients with impaired renal function due to reduced clearance.
The incidence of major bleeding by renal function status reported in clinical trials of patients receiving fondaparinux for VTE surgical prophylaxis is provided in Table 1. In these patient populations, the following is recommended:

Assess renal function periodically in patients receiving fondaparinux. Discontinue the drug immediately in patients who develop severe renal impairment while on therapy. After discontinuation of fondaparinux, its anticoagulant effects may persist for 2 to 4 days in patients with normal renal function (i.e., at least 3 to 5 half-lives). The anticoagulant effects of fondaparinux may persist even longer in patients with renal impairment.
Bleeding Risk When Body Weight < 50 Kg
Fndaparinux increases the risk for bleeding in patients who weigh less than 50 kg, compared to patients with higher weights.
In patients who weigh less than 50 kg
- Do not administer fondaparinux as prophylactic therapy for patients undergoing hip fracture, hip replacement, or knee replacement surgery and abdominal surgery.
- Use fondaparinux with caution in the treatment of PE and DVT.
During randomized clinical trials of VTE prophylaxis in the peri-operative period following hip fracture, hip replacement, or knee replacement surgery and abdominal surgery, major bleeding occurred at a higher rate among patients with a body weight <50 kg compared to those with a body weight >50 kg (5.4% versus 2.1% in patients undergoing hip fracture, hip replacement, or knee replacement surgery; 5.3% versus 3.3% in patients undergoing abdominal surgery).
Thrombocytopenia
Thrombocytopenia can occur with the administration of fondaparinux. Thrombocytopenia of any degree should be monitored closely. Discontinue fondaparinux if the platelet count falls below 100,000/mm3. Moderate thrombocytopenia(platelet counts between 100,000/mm3 and 50,000/mm3) occurred at a rate of 3.0% in patients given fondaparinux 2.5 mg in the peri-operative hip fracture, hip replacement, or knee replacement surgery and abdominal surgery clinical trials. Severe thrombocytopenia (platelet counts less than 50,000/mm3) occurred at a rate of 0.2% in patients given fondaparinux 2.5 mg in these clinical trials. During extended prophylaxis, no cases of moderate or severe thrombocytopenia were reported.
Moderate thrombocytopenia occurred at a rate of 0.5% in patients given the fondaparinux treatment regimen in the DVT and PE treatment clinical trials. Severe thrombocytopenia occurred at a rate of 0.04% in patients given the fondaparinux treatment regimen in the DVT and PE treatment clinical trials.
Isolated occurrences of thrombocytopenia with thrombosis that manifested similar to heparin-induced thrombocytopenia have been reported with the use of fondaparinux in postmarketing experience. [See Adverse Reactions (6.5)].
Neuraxial Anesthesia and Post-operative Indwelling Epidural Catheter Use
Spinal hematomas or epidural hematomas, which may result in long-term or permanent paralysis, can occur with the use of anticoagulants and neuraxial (spinal/epidural) anesthesia or spinal puncture. The risk of these events may be higher with post-operative use of indwelling epidural catheters or concomitant use of other drugs affecting hemostasis such as NSAIDs. In the postmarketing experience, epidural or spinal hematoma has been reported in association with the use of fondaparinux by subcutaneous (SC) injection. Monitor patients undergoing these procedures for signs and symptoms of neurologic impairment. Consider the potential risks and benefits before neuraxial intervention in patients anticoagulated or who may be anticoagulated for thromboprophylaxis.
Adverse Reactions
Clinical Trials Experience
The most serious adverse reactions reported with fondaparinux are bleeding complications and thrombocytopenia . Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reaction information below is based on data from 8,877 patients exposed to fondaparinux in controlled trials of hip fracture, hip replacement, major knee, or abdominal surgeries, and DVT and PE treatment. These trials consisted of the following:
- 2 peri-operative dose-response trials (n = 989)
- 4 active-controlled peri-operative VTE prophylaxis trials with enoxaparin sodium (n = 3,616), an extended VTE prophylaxis trial (n = 327), and an active-controlled trial with dalteparin sodium (n = 1,425)
- a dose-response trial (n = 111) and an active-controlled trial with enoxaparin sodium in DVT treatment (n = 1,091)
- an active-controlled trial with heparin in PE treatment (n = 1,092)
Hemorrhage
During administration of fondaparinux, the most common adverse reactions were bleeding complications.
'Hip Fracture, Hip Replacement, and Knee Replacement Surgery': The rates of major bleeding events reported during the hip fracture, hip replacement, or knee replacement surgery clinical trials with fondaparinux 2.5 mg are provided in Table 2.
b Not approved for use in patients undergoing hip fracture surgery.
c Major bleeding was defined as clinically overt bleeding that was (1) fatal, (2) bleeding at critical site (e.g. intracranial, retroperitoneal, intraocular, pericardial, spinal, or into adrenal gland), (3) associated with re-operation at operative site, or (4) with a bleeding index (BI) ≥2.
d BI ≥2: Overt bleeding associated only with a bleeding index (BI) ≥2 calculated as [number of whole blood or packed red blood cell units transfused + [(pre-bleeding) – (post-bleeding)] hemoglobin (g/dL) values].
e Minor bleeding was defined as clinically overt bleeding that was not major.
A separate analysis of major bleeding across all randomized, controlled, peri-operative, prophylaxis clinical studies of hip fracture, hip replacement, or knee replacement surgery according to the time of the first injection of fondaparinux after surgical closure was performed in patients who received fondaparinux only post-operatively. In this analysis, the incidences of major bleeding were as follows: <4 hours was 4.8% (5/104), 4 to 6 hours was 2.3% (28/1,196), 6 to 8 hours was 1.9% (38/1,965). In all studies, the majority (≥75%) of the major bleeding events occurred during the first 4 days after surgery.
Abdominal Surgery: In a randomized study of patients undergoing abdominal surgery, fondaparinux 2.5 mg once daily (n = 1,433) was compared with dalteparin 5,000 IU once daily (n = 1,425). Bleeding rates are shown in Table 3.
b Minor bleeding was defined as clinically overt bleeding that was not major.
The rates of major bleeding according to the time interval following the first fondaparinux injection were as follows: <6 hours was 3.4% (9/263) and 6 to 8 hours was 2.9% (32/1112).
Treatment of Deep Vein Thrombosis and Pulmonary Embolism: The rates of bleeding events reported during the DVT and PE clinical trials with the fondaparinux injection treatment regimen are provided in Table 4.
b Major bleeding was defined as clinically overt: –and/or contributing to death – and/or in a critical organ including intracranial, retroperitoneal, intraocular, spinal, pericardial, or adrenal gland – and/or associated with a fall in hemoglobin level ≥2 g/dL – and/or leading to a transfusion ≥2 units of packed red blood cells or whole blood.
c Clinically overt bleeding with a 2 g/dL fall in hemoglobin and/or leading to transfusion of PRBC or whole blood ≥2 units.
d Minor bleeding was defined as clinically overt bleeding that was not major.
Local Reactions
Local irritation (injection site bleeding, rash, and pruritus) may occur following subcutaneous injection of fondaparinux.
Elevations of Serum Aminotransferases
In the peri-operative prophylaxis randomized clinical trials of 7 ± 2 days, asymptomatic increases in aspartate (AST) and alanine (ALT) aminotransferase levels greater than 3 times the upper limit of normal were reported in 1.7% and 2.6% of patients, respectively, during treatment with fondaparinux 2.5 mg once daily versus 3.2% and 3.9% of patients, respectively, during treatment with enoxaparin sodium 30 mg every 12 hours or 40 mg once daily enoxaparin sodium. These elevations are reversible and rarely associated with increases in bilirubin. In the extended prophylaxis clinical trial, no significant differences in AST and ALT levels between fondaparinux 2.5 mg and placebo-treated patients were observed.
In the DVT and PE treatment clinical trials, asymptomatic increases in AST and ALT levels greater than 3 times the upper limit of normal of the laboratory reference range were reported in 0.7% and 1.3% of patients, respectively, during treatment with fondaparinux. In comparison, these increases were reported in 4.8% and 12.3% of patients, respectively, in the DVT treatment trial during treatment with enoxaparin sodium 1 mg/kg every 12 hours and in 2.9% and 8.7% of patients, respectively, in the PE treatment trial during treatment with aPTT adjusted heparin.
Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, elevations that might be caused by drugs like fondaparinux should be interpreted with caution.
Other Adverse Reactions
Other adverse reactions that occurred during treatment with fondaparinux in clinical trials with patients undergoing hip fracture, hip replacement, or knee replacement surgery are provided in Table 5.
b Not approved for use in patients undergoing hip fracture surgery.
c Localized blister coded as bullous eruption.
Adverse reactions in the abdominal surgery study and in the VTE treatment trials generally occurred at lower rates than in the hip and knee surgery trials described above. The most common adverse reaction in the abdominal surgery trial was post-operative wound infection (4.9%), and the most common adverse reaction in the VTE treatment trials was epistaxis(1.3%).
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fondaparinux. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Isolated occurrences of thrombocytopenia with thrombosis that manifested similar to heparin-induced thrombocytopenia have been reported in the postmarketing experience and isolated cases of elevated aPTT temporally associated with bleeding events have been reported following administration of fondaparinux (with or without concomitant administration of other anticoagulants).
Serious allergic reactions, including angioedema, anaphylactoid/anaphylactic reactions have been reported with the use of fondaparinux.
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
No dose adjustment is recommended in patients with mild to moderate hepatic impairment, based upon single-dose pharmacokinetic data. Pharmacokinetic data are not available for patients with severe hepatic impairment. Patients with hepatic impairment may be particularly vulnerable to bleeding during Fondaparinux therapy. Observe these patients closely for signs and symptoms of bleeding.
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
- Fondaparinux injection is provided in a single-dose, prefilled syringe affixed with an automatic needle protection system.
- Fondaparinux is administered by subcutaneous injection. It must not be administered by intramuscular injection.
- Fondaparinux is intended for use under a physician’s guidance.
- Patients may self-inject only if their physician determines that it is appropriate and the patients are trained in subcutaneous injection techniques.
- Prior to administration, visually inspect fondaparinux to ensure the solution is clear and free of particulate matter.
- To avoid the loss of drug when using the prefilled syringe, do not expel the air bubble from the syringe before the injection.
- Administration should be made in the fatty tissue, alternating injection sites (e.g., between the left and right anterolateral or the left and right posterolateral abdominal wall).
Monitoring
Routine coagulation tests such as prothrombin time (PT) and activated partial thromboplastin time (aPTT) are relatively insensitive measures of the activity of fondaparinux and international standards of heparin or LMWH are not calibrators to measure anti-Factor Xa activity of fondaparinux. If unexpected changes in coagulation parameters or major bleeding occur during therapy with fondaparinux, discontinue fondaparinux. In postmarketing experience, isolated occurrences of aPTT elevations have been reported following administration of fondaparinux.
Periodic routine complete blood counts (including platelet count), serum creatinine level, and stool occult blood test are recommended during the course of treatment with fondaparinux.
The anti-factor Xa activity of fondaparinux sodium can be measured by anti-Xa assay using the appropriate calibrator (fondaparinux). The activity of fondaparinux sodium is expressed in milligrams (mg) of the fondaparinux and cannot be compared with activities of heparin or low molecular weight heparins.
IV Compatibility
There is limited information regarding the compatibility of Fondaparinux and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
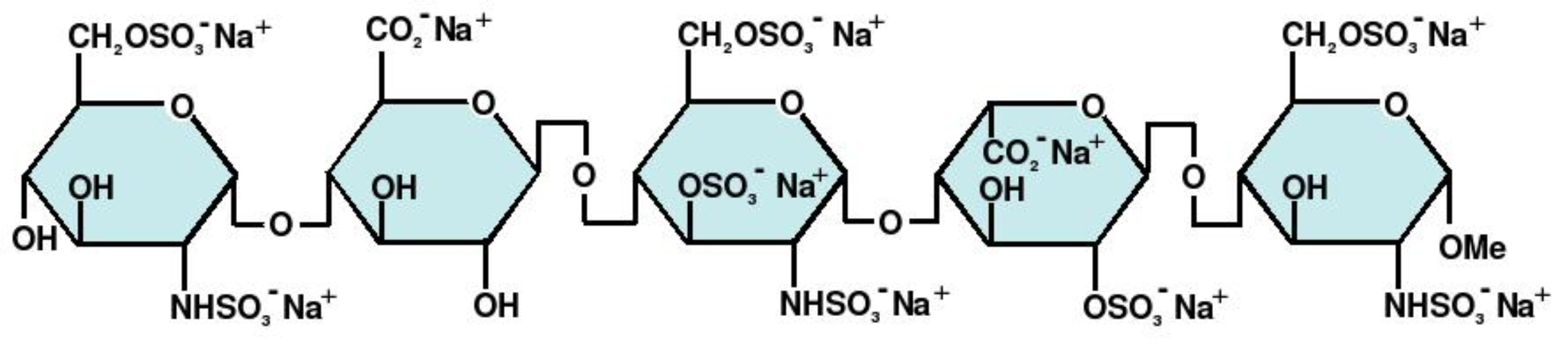
| |
Fondaparinux
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Fondaparinux Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Fondaparinux |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Fondaparinux |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Fondaparinux interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Fondaparinux Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.