Flurazepam hydrochloride: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
*Initial dose is 15 mg for women and either 15 mg or 30 mg for men. | *Initial dose is 15 mg for women and either 15 mg or 30 mg for men. | ||
*The 15 mg dose can be increased to 30 mg if necessary for efficacy. | *The 15 mg dose can be increased to 30 mg if necessary for efficacy. | ||
*In elderly dosage should be limited to 15 mg. | *In elderly dosage should be limited to 15 mg. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Flurazepam hydrochloride in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Flurazepam hydrochloride in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Flurazepam hydrochloride in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Flurazepam hydrochloride in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Flurazepam hydrochloride in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Flurazepam hydrochloride in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Flurazepam hydrochloride in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Flurazepam hydrochloride in pediatric patients. | ||
|contraindications=*In patients with known hypersensitivity to flurazepam or other benzodiazepines. | |contraindications=*In patients with known hypersensitivity to flurazepam or other benzodiazepines. | ||
|warnings=====CNS-Depressant Effects and Daytime Impairment==== | |warnings=====CNS-Depressant Effects and Daytime Impairment==== | ||
[[Dizziness]], [[drowsiness]], light-headedness, staggering, ataxia and falling can occur, particularly in elderly or debilitated persons. Severe sedation, lethargy, disorientation and coma, probably indicative of drug intolerance or overdosage, have been reported. | *[[Dizziness]], [[drowsiness]], [[light-headedness]], [[staggering]], [[ataxia]] and [[falling]] can occur, particularly in elderly or debilitated persons. | ||
*Severe [[sedation]], [[lethargy]], [[disorientation]] and [[coma]], probably indicative of drug intolerance or overdosage, have been reported. | |||
*Flurazepam is a [[central nervous system]] ([[CNS]]) depressant and can impair daytime function even when used as prescribed. | |||
*Prescribers should monitor for excess depressant effects, but impairment can occur in the absence of subjective symptoms, and may not be reliably detected by ordinary clinical exam (i.e., less than formal psychomotor testing). While pharmacodynamic tolerance or adaptation to some adverse depressant effects of flurazepam may develop, patients using flurazepam should be cautioned against driving or engaging in other hazardous activities or activities requiring complete mental alertness. | |||
*Additive effects occur with concomitant use of other CNS depressants (e.g., other [[benzodiazepines]], [[opioids]], [[tricyclic antidepressants]], [[alcohol]]). | |||
*Downward dose adjustment of flurazepam and concomitant CNS depressants should be considered. | |||
*The potential for adverse drug interactions continues for several days following discontinuation of flurazepam, until serum levels of psychoactive metabolites decline. | |||
*Use of flurazepam with other [[sedative]]-[[hypnotics]] is not recommended. *[[Alcohol]] generally should not be used during treatment with flurazepam. | |||
*The risk of next-day psychomotor impairment is increased if flurazepam is taken with less than a full night of sleep remaining (7 to 8 hours); if higher than the recommended dose is taken; if coadministered with other [[CNS depressants]]. | |||
====Benzodiazepine Withdrawal Syndrome==== | |||
*[[Withdrawal]] symptoms of the [[barbiturate]] type have occurred after the discontinuation of [[benzodiazepines]]. | |||
====Need to Evaluate for Co-morbid Disorders==== | |||
*Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of [[insomnia]] should be initiated only after a careful evaluation of the patient. | |||
*The failure of [[insomnia]] to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. | |||
*Worsening of [[insomnia]] or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with [[sedative]]-[[hypnotic]] drugs. | |||
====Severe Anaphylactic or Anaphylactoid Reactions==== | |||
*Rare cases of [[angioedema]] involving the [[tongue]], [[glottis]] or [[larynx]] have been reported in patients after taking the first or subsequent doses of [[sedative]]-[[hypnotics]], including flurazepam. | |||
*Some patients have had additional symptoms such as [[dyspnea]], throat closing, or [[nausea]] and [[vomiting]] that suggest [[anaphylaxis]]. | |||
*Some patients have required medical therapy in the emergency department. | |||
*If [[angioedema]] involves the [[tongue]], [[glottis]] or [[larynx]], [[airway obstruction]] may occur and be fatal. | |||
*Patients who develop [[angioedema] after treatment with flurazepam should not be rechallenged with the drug. | |||
====Abnormal Thinking and Behavior Changes==== | |||
*Abnormal thinking and behavior changes have been reported in patients treated with [[sedative]]-[[hypnotics]] including flurazepam. | |||
*Some of these changes include decreased inhibition (e.g., aggressiveness and extroversion that seemed out of character), bizarre behavior, and [[depersonalization]]. | |||
*Visual and auditory [[hallucinations]] have also been reported. [[Amnesia]], and other neuro-psychiatric symptoms, may occur. | |||
*Paradoxical reactions such as stimulation, agitation, increased muscle spasticity, and sleep disturbances may occur unpredictably. | |||
*Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake, with [[amnesia]] for the event) have been reported with use of [[sedative]]-[[hypnotics]]. | |||
*These behaviors can occur with initial treatment or in patients previously tolerant of flurazepam or other [[sedative]]-[[hypnotics]]. Although these behaviors can occur with use at therapeutic doses, risk is increased by higher doses or concomitant use of [[alcohol]] or other CNS depressants. Due to risk to the patient and community, flurazepam should be discontinued if “sleep-driving” occurs. | |||
*Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. | |||
*As with sleep-driving, patients usually do not remember these events. | |||
5. | 5.6 Worsening of Depression | ||
*[[Benzodiazepines]] may worsen [[depression]]. | |||
*Consequently, appropriate precautions (e.g., limiting the total prescription size and increased monitoring for suicidal ideation) should be considered. | |||
|clinicalTrials=====General==== | |||
*[[Weakness]] | |||
*[[Irritability]] | |||
====Nervous system==== | |||
*[[Headache]] | |||
====Digestive system==== | |||
*[[Heartburn]] | |||
*Upset stomach | |||
*[[Nausea]] | |||
*[[Vomiting]] | |||
*[[Diarrhea]] | |||
*[[Constipation]] | |||
*Gastrointestinal pain | |||
====Psichiatric disorders==== | |||
*[[Nervousness]] | |||
*Talkativeness | |||
*Apprehension | |||
====Cardiovascular system==== | |||
*[[Palpitations]] | |||
====Others==== | |||
*Chest pains | |||
*Body and joint pains | |||
*Genitourinary complaints | |||
There have also been rare occurrences of: | |||
*[[Leukopenia]] | |||
*[[Granulocytopenia]] | |||
*[[Sweating]] | |||
*[[Flushes]] | |||
*Difficulty in focusing | |||
*[[Blurred vision]] | |||
*Burning eyes | |||
|drugInteractions=Benzodiazepines, including flurazepam, produce additive CNS depressant effects when co-administered with ethanol or other CNS depressants (e.g., psychotropic medications, anticonvulsants, antihistamines). Downward dose adjustment of flurazepam and/or concomitant CNS depressants may be necessary because of additive effects. | *[[Faintness]] | ||
*[[Hypotension]] | |||
*Shortness of breath | |||
*[[Pruritus]] | |||
*Skin rash | |||
*[[Dry mouth]] | |||
*Bitter taste | |||
*[[Excessive salivation]] | |||
*[[Anorexia]] | |||
*[[Euphoria]] | |||
*[[Depression]] | |||
*[[Slurred speech]] | |||
*[[Confusion]] | |||
*[[Restlessness]] | |||
*[[Hallucinations]] | |||
*Elevated SGOT, SGPT, total and direct [[bilirubin]] elevations, and elevated [[alkaline phosphatase]]. | |||
|drugInteractions=*[[Benzodiazepines]], including flurazepam, produce additive [[CNS]] depressant effects when co-administered with [[ethanol]] or other [[CNS]] depressants (e.g., psychotropic medications, [[anticonvulsants]], [[antihistamines]]). | |||
*Downward dose adjustment of flurazepam and/or concomitant [[CNS]] depressants may be necessary because of additive effects. | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=Teratogenic Effects | |useInPregnancyFDA=====Teratogenic Effects==== | ||
*There are no adequate and well-controlled studies in pregnant women. | |||
There are no adequate and well-controlled studies in pregnant women. Available human data on the risk of teratogenicity for benzodiazepines are inconclusive. There is insufficient evidence in humans to assess the effect of benzodiazepine exposure during pregnancy or neurodevelopment. Administration of benzodiazepines immediately prior to or during childbirth can result in a syndrome of hypothermia, hypotonia, respiratory depression, and difficulty feeding. In addition, infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period. Administration of flurazepam to pregnant animals did not indicate a risk for adverse effects on morphological development at clinically relevant doses; however, animal data for other benzodiazepines suggest that possibility of adverse developmental effects (including long-term effects on neurobehavioral and immunological function) following prenatal exposure. Flurazepam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | *Available human data on the risk of [[teratogenicity]] for [[benzodiazepines]] are inconclusive. | ||
*There is insufficient evidence in humans to assess the effect of [[benzodiazepine]] exposure during pregnancy or neurodevelopment. *Administration of [[benzodiazepines]] immediately prior to or during childbirth can result in a syndrome of [[hypothermia]], [[hypotonia]], respiratory depression, and difficulty feeding. In addition, infants born to mothers who have taken [[benzodiazepines]] during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period. | |||
*Administration of flurazepam to pregnant animals did not indicate a risk for adverse effects on morphological development at clinically relevant doses; however, animal data for other [[benzodiazepines]] suggest that possibility of adverse developmental effects (including long-term effects on neurobehavioral and immunological function) following prenatal exposure. | |||
*Flurazepam should be used during pregnancy only if the potential benefit justifies the potential risk to the [[fetus]]. | |||
|AUSPregCat=C | |AUSPregCat=C | ||
|useInPed=Safety and effectiveness in pediatric patients have not been established. | |useInPed=*Safety and effectiveness in pediatric patients have not been established. | ||
|useInGeri=Flurazepam may cause confusion and over-sedation in the elderly. Elderly patients generally should be started on a low dose of flurazepam and observed closely. | |useInGeri=*Flurazepam may cause confusion and over-sedation in the elderly. Elderly patients generally should be started on a low dose of flurazepam and observed closely. | ||
*Elderly or debilitated patients may be more sensitive to [[benzodiazepines]], reflecting the greater frequency of decreased [[hepatic]], [[renal]], or [[cardiac]] function, and of concomitant disease or other drug therapy. | |||
Elderly or debilitated patients may be more sensitive to benzodiazepines, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender=*Flurazepam clearance is lower in women | |useInGender=*Flurazepam clearance is lower in women | ||
|overdose=Manifestations of flurazepam hydrochloride overdosage include somnolence, confusion and coma. Respiration, pulse and blood pressure should be monitored as in all cases of drug overdosage. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension and CNS depression may be combated by judicious use of appropriate therapeutic agents. The value of dialysis has not been determined. If excitation occurs in patients following flurazepam hydrochloride overdosage, barbiturates should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested. | |overdose=*Manifestations of flurazepam hydrochloride overdosage include [[somnolence]], [[confusion]] and [[coma]]. | ||
*Respiration, pulse and blood pressure should be monitored as in all cases of drug overdosage. | |||
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be useful in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use. | *General supportive measures should be employed, along with immediate [[gastric lavage]]. | ||
*Intravenous fluids should be administered and an adequate airway maintained. | |||
*[[Hypotension]] and [[CNS]] depression may be combated by judicious use of appropriate therapeutic agents. The value of dialysis has not been determined. *If excitation occurs in patients following flurazepam hydrochloride overdosage, [[barbiturates]] should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested. | |||
*Flumazenil, a specific [[benzodiazepine]]-receptor [[antagonist]], is indicated for the complete or partial reversal of the sedative effects of [[benzodiazepines]] and may be useful in situations when an overdose with a [[benzodiazepine]] is known or suspected. | |||
*Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. | |||
*Flumazenil is intended as an adjunct to, not as a substitute for, proper management of [[benzodiazepine]] overdose. | |||
*Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual [[benzodiazepine]] effects for an appropriate period after treatment. | |||
*The prescriber should be aware of a risk of [[seizure]] in association with flumazenil treatment, particularly in long-term [[benzodiazepine]] users and in cyclic antidepressant overdose. | |||
*The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use. | |||
|mechAction=Flurazepam, like other central nervous system agents of the 1,4-benzodiazepine class, presumably exerts its effects by binding to stereo-specific receptors at several sites within the central nervous system (CNS). The exact mechanism of action is unknown. | |mechAction=Flurazepam, like other central nervous system agents of the 1,4-benzodiazepine class, presumably exerts its effects by binding to stereo-specific receptors at several sites within the central nervous system (CNS). The exact mechanism of action is unknown. | ||
|structure=Flurazepam hydrochloride is chemically 7-chloro-1-[2-(diethylamino)ethyl]-5-(o-fluoro-phenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one dihydrochloride. It is a pale yellow, crystalline compound, freely soluble in alcohol and very soluble in water. It has a molecular weight of 460.81 and the following structural formula: | |structure=Flurazepam hydrochloride is chemically 7-chloro-1-[2-(diethylamino)ethyl]-5-(o-fluoro-phenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one dihydrochloride. It is a pale yellow, crystalline compound, freely soluble in alcohol and very soluble in water. It has a molecular weight of 460.81 and the following structural formula: | ||
| Line 74: | Line 142: | ||
This pharmacokinetic profile may be responsible for the clinical observation that flurazepam is increasingly effective on the second or third night of consecutive use and that for 1 or 2 nights after the drug is discontinued both sleep latency and total wake time may still be decreased. | This pharmacokinetic profile may be responsible for the clinical observation that flurazepam is increasingly effective on the second or third night of consecutive use and that for 1 or 2 nights after the drug is discontinued both sleep latency and total wake time may still be decreased. | ||
The single dose pharmacokinetics of flurazepam were studied in 12 healthy geriatric subjects (aged 61 to 85 years). The mean elimination half-life of desalkyl-flurazepam was longer in elderly male subjects (160 hours) compared with younger male subjects (74 hours), while mean elimination half-life was similar in geriatric female subjects (120 hours) and younger female subjects (90 hours). After multiple dosing, mean steady-state plasma levels of desalkyl-flurazepam were higher in elderly male subjects (81 ng/mL) compared with younger male subjects (53 ng/mL), while values were similar between elderly female subjects (85 ng/mL) and younger female subjects (86 ng/mL). The mean washout half-life of desalkyl-flurazepam was longer in elderly male and female subjects (126 and 158 hours, respectively) compared with younger male and female subjects (111 and 113 hours, respectively) | The single dose pharmacokinetics of flurazepam were studied in 12 healthy geriatric subjects (aged 61 to 85 years). The mean elimination half-life of desalkyl-flurazepam was longer in elderly male subjects (160 hours) compared with younger male subjects (74 hours), while mean elimination half-life was similar in geriatric female subjects (120 hours) and younger female subjects (90 hours). After multiple dosing, mean steady-state plasma levels of desalkyl-flurazepam were higher in elderly male subjects (81 ng/mL) compared with younger male subjects (53 ng/mL), while values were similar between elderly female subjects (85 ng/mL) and younger female subjects (86 ng/mL). The mean washout half-life of desalkyl-flurazepam was longer in elderly male and female subjects (126 and 158 hours, respectively) compared with younger male and female subjects (111 and 113 hours, respectively). | ||
|nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility | |nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | ||
Studies to assess the genotoxic or carcinogenic potential of flurazepam or the effects of flurazepam on fertility have not been conducted. | |||
|clinicalStudies=*Sleep laboratory studies have objectively determined that flurazepam hydrochloride capsules are effective for at least 28 consecutive nights of drug administration. | |||
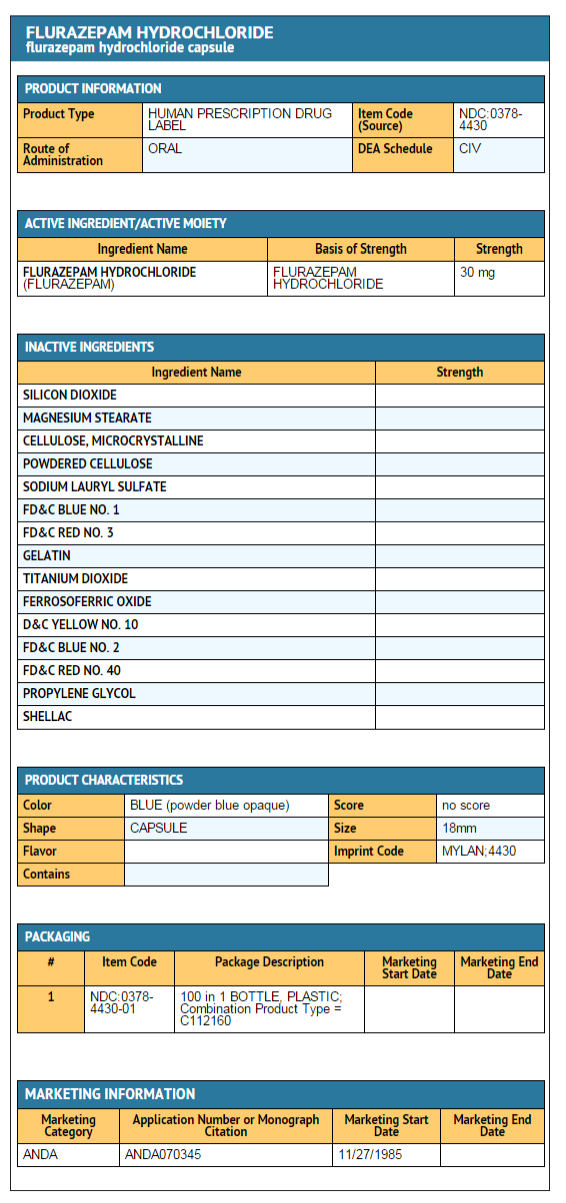
|howSupplied=====15 mg capsule==== | |||
*Is a hard-shell gelatin capsule with a white opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4415 in black ink on both the cap and body. | |||
*They are available as follows: | |||
NDC 0378-4415-01 | |||
Bottles of 100 capsules | |||
====30 mg capsule==== | |||
*Is a hard-shell gelatin capsule with a powder blue opaque cap and a powder blue opaque body filled with off-white to yellow powder. | |||
*The capsule is axially printed with MYLAN over 4430 in black ink on both the cap and body. | |||
They are available as follows: | |||
NDC 0378-4430-01 | |||
| | bottles of 100 capsules | ||
|storage=*Store at 20° to 25°C (68° to 77°F). | |||
*Protect from light. | |||
*Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. | |||
|packLabel=[[File:Flurazepam FDA label 15mg.png|none|450px]] | |packLabel=[[File:Flurazepam FDA label 15mg.png|none|450px]] | ||
[[File:Flurazepam FDA label 30mg.png|none|450px]] | [[File:Flurazepam FDA label 30mg.png|none|450px]] | ||
|fdaPatientInfo=Inform patients about the benefits and risks of flurazepam, stressing the importance of use as directed. Assist patients in understanding the Medication Guide and instruct them to read it with each prescription refill. | |fdaPatientInfo=Inform patients about the benefits and risks of flurazepam, stressing the importance of use as directed. Assist patients in understanding the Medication Guide and instruct them to read it with each prescription refill. | ||
CNS Depressant Effects and Next-Day Impairment | ====CNS Depressant Effects and Next-Day Impairment==== | ||
Tell patients that flurazepam can cause next-day impairment, even in the absence of symptoms. Caution patients against driving or engaging in other hazardous activities or activities requiring complete mental alertness when using flurazepam. Tell patients that daytime impairment may persist for several days following discontinuation of flurazepam. | Tell patients that flurazepam can cause next-day impairment, even in the absence of symptoms. Caution patients against driving or engaging in other hazardous activities or activities requiring complete mental alertness when using flurazepam. Tell patients that daytime impairment may persist for several days following discontinuation of flurazepam. | ||
Withdrawal | ====Withdrawal==== | ||
Instruct patients to contact you before stopping or decreasing the dose of flurazepam, because [[withdrawal]] symptoms can occur. | |||
Instruct patients to contact you before stopping or decreasing the dose of flurazepam, because withdrawal symptoms can occur | |||
====Abnormal Thinking and Behavior Change==== | |||
Instruct patients that [[sedative]] [[hypnotics]] can cause abnormal thinking and behavior change, including “sleepdriving” and other complex behaviors while not being fully awake (preparing and eating food, making phone calls, or having sex). Tell patients to call you immediately if they develop any of these symptoms. | |||
====Severe Allergic Reactions==== | |||
Inform patients that severe allergic reactions can occur from flurazepam. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if these occur. | Inform patients that severe allergic reactions can occur from flurazepam. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if these occur. | ||
Suicide | ====Suicide==== | ||
Tell patients that flurazepam can worsen depression, and to immediately report any suicidal thoughts. | Tell patients that flurazepam can worsen depression, and to immediately report any suicidal thoughts. | ||
Alcohol and Other Drugs | ====Alcohol and Other Drugs==== | ||
Ask patients about [[alcohol]] consumption, medicines they are taking now, and drugs they may be taking without a prescription. Advise patients that [[alcohol]] generally should not be used during treatment with flurazepam. | |||
Ask patients about alcohol consumption, medicines they are taking now, and drugs they may be taking without a prescription. Advise patients that alcohol generally should not be used during treatment with flurazepam. | |||
====Pregnancy==== | |||
Instruct patients to inform you if they are nursing or pregnant, or may become pregnant while taking flurazepam. If a woman becomes pregnant while taking flurazepam, she should discontinue use immediately. | Instruct patients to inform you if they are nursing or pregnant, or may become pregnant while taking flurazepam. If a woman becomes pregnant while taking flurazepam, she should discontinue use immediately. | ||
Tolerance, Abuse, and Dependence | ====Tolerance, Abuse, and Dependence==== | ||
Tell patients not to increase the dose of flurazepam on their own, and to inform you if they believe the drug “does not work”. | Tell patients not to increase the dose of flurazepam on their own, and to inform you if they believe the drug “does not work”. | ||
|alcohol=*Additive effects occur with concomitant use of alcohol. | |alcohol=*Additive effects occur with concomitant use of alcohol. | ||
*Sleep driving and other complex behaviors while not fully awake: Risk increases with dose and concomitant CNS depressants and alcohol. | *Sleep driving and other complex behaviors while not fully awake: Risk increases with dose and concomitant [[CNS]] depressants and [[alcohol]]. | ||
|brandNames=*Dalmane | |brandNames=*Dalmane<ref>{{cite web|url=http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2f2db2f5-49d3-4d47-a08a-628df49d2120|title= FLURAZEPAM HYDROCHLORIDE- flurazepam hydrochloride capsule}} </ref> | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
Revision as of 03:14, 22 January 2015
{{DrugProjectFormSinglePage |authorTag=Stefano Giannoni [1] |genericName=Flurazepam hydrochloride |aOrAn=a |drugClass=Benzodiazepine |indicationType=treatment |indication=insomnia |adverseReactions=dizziness, drowsiness, light-headedness, staggering, ataxia. |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult=====Insomnia====
- Initial dose is 15 mg for women and either 15 mg or 30 mg for men.
- The 15 mg dose can be increased to 30 mg if necessary for efficacy.
- In elderly dosage should be limited to 15 mg.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Flurazepam hydrochloride in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Flurazepam hydrochloride in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Flurazepam hydrochloride in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Flurazepam hydrochloride in pediatric patients. |contraindications=*In patients with known hypersensitivity to flurazepam or other benzodiazepines. |warnings=====CNS-Depressant Effects and Daytime Impairment====
- Dizziness, drowsiness, light-headedness, staggering, ataxia and falling can occur, particularly in elderly or debilitated persons.
- Severe sedation, lethargy, disorientation and coma, probably indicative of drug intolerance or overdosage, have been reported.
- Flurazepam is a central nervous system (CNS) depressant and can impair daytime function even when used as prescribed.
- Prescribers should monitor for excess depressant effects, but impairment can occur in the absence of subjective symptoms, and may not be reliably detected by ordinary clinical exam (i.e., less than formal psychomotor testing). While pharmacodynamic tolerance or adaptation to some adverse depressant effects of flurazepam may develop, patients using flurazepam should be cautioned against driving or engaging in other hazardous activities or activities requiring complete mental alertness.
- Additive effects occur with concomitant use of other CNS depressants (e.g., other benzodiazepines, opioids, tricyclic antidepressants, alcohol).
- Downward dose adjustment of flurazepam and concomitant CNS depressants should be considered.
- The potential for adverse drug interactions continues for several days following discontinuation of flurazepam, until serum levels of psychoactive metabolites decline.
- Use of flurazepam with other sedative-hypnotics is not recommended. *Alcohol generally should not be used during treatment with flurazepam.
- The risk of next-day psychomotor impairment is increased if flurazepam is taken with less than a full night of sleep remaining (7 to 8 hours); if higher than the recommended dose is taken; if coadministered with other CNS depressants.
Benzodiazepine Withdrawal Syndrome
- Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines.
Need to Evaluate for Co-morbid Disorders
- Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient.
- The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated.
- Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs.
Severe Anaphylactic or Anaphylactoid Reactions
- Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including flurazepam.
- Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis.
- Some patients have required medical therapy in the emergency department.
- If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal.
- Patients who develop [[angioedema] after treatment with flurazepam should not be rechallenged with the drug.
Abnormal Thinking and Behavior Changes
- Abnormal thinking and behavior changes have been reported in patients treated with sedative-hypnotics including flurazepam.
- Some of these changes include decreased inhibition (e.g., aggressiveness and extroversion that seemed out of character), bizarre behavior, and depersonalization.
- Visual and auditory hallucinations have also been reported. Amnesia, and other neuro-psychiatric symptoms, may occur.
- Paradoxical reactions such as stimulation, agitation, increased muscle spasticity, and sleep disturbances may occur unpredictably.
- Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake, with amnesia for the event) have been reported with use of sedative-hypnotics.
- These behaviors can occur with initial treatment or in patients previously tolerant of flurazepam or other sedative-hypnotics. Although these behaviors can occur with use at therapeutic doses, risk is increased by higher doses or concomitant use of alcohol or other CNS depressants. Due to risk to the patient and community, flurazepam should be discontinued if “sleep-driving” occurs.
- Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic.
- As with sleep-driving, patients usually do not remember these events.
5.6 Worsening of Depression
- Benzodiazepines may worsen depression.
- Consequently, appropriate precautions (e.g., limiting the total prescription size and increased monitoring for suicidal ideation) should be considered.
|clinicalTrials=====General====
Nervous system
Digestive system
- Heartburn
- Upset stomach
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Gastrointestinal pain
Psichiatric disorders
- Nervousness
- Talkativeness
- Apprehension
Cardiovascular system
Others
- Chest pains
- Body and joint pains
- Genitourinary complaints
There have also been rare occurrences of:
- Leukopenia
- Granulocytopenia
- Sweating
- Flushes
- Difficulty in focusing
- Blurred vision
- Burning eyes
- Faintness
- Hypotension
- Shortness of breath
- Pruritus
- Skin rash
- Dry mouth
- Bitter taste
- Excessive salivation
- Anorexia
- Euphoria
- Depression
- Slurred speech
- Confusion
- Restlessness
- Hallucinations
- Elevated SGOT, SGPT, total and direct bilirubin elevations, and elevated alkaline phosphatase.
|drugInteractions=*Benzodiazepines, including flurazepam, produce additive CNS depressant effects when co-administered with ethanol or other CNS depressants (e.g., psychotropic medications, anticonvulsants, antihistamines).
- Downward dose adjustment of flurazepam and/or concomitant CNS depressants may be necessary because of additive effects.
|FDAPregCat=C |useInPregnancyFDA=====Teratogenic Effects====
- There are no adequate and well-controlled studies in pregnant women.
- Available human data on the risk of teratogenicity for benzodiazepines are inconclusive.
- There is insufficient evidence in humans to assess the effect of benzodiazepine exposure during pregnancy or neurodevelopment. *Administration of benzodiazepines immediately prior to or during childbirth can result in a syndrome of hypothermia, hypotonia, respiratory depression, and difficulty feeding. In addition, infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period.
- Administration of flurazepam to pregnant animals did not indicate a risk for adverse effects on morphological development at clinically relevant doses; however, animal data for other benzodiazepines suggest that possibility of adverse developmental effects (including long-term effects on neurobehavioral and immunological function) following prenatal exposure.
- Flurazepam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
|AUSPregCat=C |useInPed=*Safety and effectiveness in pediatric patients have not been established. |useInGeri=*Flurazepam may cause confusion and over-sedation in the elderly. Elderly patients generally should be started on a low dose of flurazepam and observed closely.
- Elderly or debilitated patients may be more sensitive to benzodiazepines, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
|useInGender=*Flurazepam clearance is lower in women |overdose=*Manifestations of flurazepam hydrochloride overdosage include somnolence, confusion and coma.
- Respiration, pulse and blood pressure should be monitored as in all cases of drug overdosage.
- General supportive measures should be employed, along with immediate gastric lavage.
- Intravenous fluids should be administered and an adequate airway maintained.
- Hypotension and CNS depression may be combated by judicious use of appropriate therapeutic agents. The value of dialysis has not been determined. *If excitation occurs in patients following flurazepam hydrochloride overdosage, barbiturates should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested.
- Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be useful in situations when an overdose with a benzodiazepine is known or suspected.
- Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access.
- Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose.
- Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment.
- The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.
- The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
|mechAction=Flurazepam, like other central nervous system agents of the 1,4-benzodiazepine class, presumably exerts its effects by binding to stereo-specific receptors at several sites within the central nervous system (CNS). The exact mechanism of action is unknown. |structure=Flurazepam hydrochloride is chemically 7-chloro-1-[2-(diethylamino)ethyl]-5-(o-fluoro-phenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one dihydrochloride. It is a pale yellow, crystalline compound, freely soluble in alcohol and very soluble in water. It has a molecular weight of 460.81 and the following structural formula:
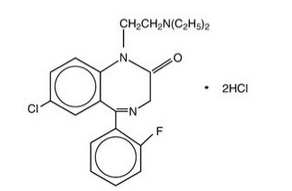
|PK=Flurazepam hydrochloride is rapidly absorbed from the gastro-intestinal tract. Flurazepam is rapidly metabolized and is excreted primarily in the urine. Following a single oral dose, peak flurazepam plasma concentrations ranging from 0.5 to 4.0 ng/mL occur at 30 to 60 minutes post-dosing. The harmonic mean apparent half-life of flurazepam is 2.3 hours. The blood level profile of flurazepam and its major metabolites was determined in man following the oral administration of 30 mg daily for 2 weeks. The N1-hydroxyethyl-flurazepam was measurable only during the early hours after a 30 mg dose and was not detectable after 24 hours. The major metabolite in blood was N1-desalkyl-flurazepam, which reached steady-state (plateau) levels after 7 to 10 days of dosing, at levels approximately 5- to 6-fold greater than the 24-hour levels observed on Day 1. The half-life of elimination of N1-desalkyl-flurazepam ranged from 47 to 100 hours. The major urinary metabolite is conjugated N1-hydroxyethyl-flurazepam which accounts for 22% to 55% of the dose. Less than 1% of the dose is excreted in the urine as N1-desalkyl-flurazepam.
This pharmacokinetic profile may be responsible for the clinical observation that flurazepam is increasingly effective on the second or third night of consecutive use and that for 1 or 2 nights after the drug is discontinued both sleep latency and total wake time may still be decreased.
The single dose pharmacokinetics of flurazepam were studied in 12 healthy geriatric subjects (aged 61 to 85 years). The mean elimination half-life of desalkyl-flurazepam was longer in elderly male subjects (160 hours) compared with younger male subjects (74 hours), while mean elimination half-life was similar in geriatric female subjects (120 hours) and younger female subjects (90 hours). After multiple dosing, mean steady-state plasma levels of desalkyl-flurazepam were higher in elderly male subjects (81 ng/mL) compared with younger male subjects (53 ng/mL), while values were similar between elderly female subjects (85 ng/mL) and younger female subjects (86 ng/mL). The mean washout half-life of desalkyl-flurazepam was longer in elderly male and female subjects (126 and 158 hours, respectively) compared with younger male and female subjects (111 and 113 hours, respectively). |nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== Studies to assess the genotoxic or carcinogenic potential of flurazepam or the effects of flurazepam on fertility have not been conducted. |clinicalStudies=*Sleep laboratory studies have objectively determined that flurazepam hydrochloride capsules are effective for at least 28 consecutive nights of drug administration. |howSupplied=====15 mg capsule====
- Is a hard-shell gelatin capsule with a white opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4415 in black ink on both the cap and body.
- They are available as follows:
NDC 0378-4415-01 Bottles of 100 capsules
30 mg capsule
- Is a hard-shell gelatin capsule with a powder blue opaque cap and a powder blue opaque body filled with off-white to yellow powder.
- The capsule is axially printed with MYLAN over 4430 in black ink on both the cap and body.
They are available as follows:
NDC 0378-4430-01 bottles of 100 capsules |storage=*Store at 20° to 25°C (68° to 77°F).
- Protect from light.
- Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
|packLabel=

|fdaPatientInfo=Inform patients about the benefits and risks of flurazepam, stressing the importance of use as directed. Assist patients in understanding the Medication Guide and instruct them to read it with each prescription refill.
CNS Depressant Effects and Next-Day Impairment
Tell patients that flurazepam can cause next-day impairment, even in the absence of symptoms. Caution patients against driving or engaging in other hazardous activities or activities requiring complete mental alertness when using flurazepam. Tell patients that daytime impairment may persist for several days following discontinuation of flurazepam.
Withdrawal
Instruct patients to contact you before stopping or decreasing the dose of flurazepam, because withdrawal symptoms can occur.
Abnormal Thinking and Behavior Change
Instruct patients that sedative hypnotics can cause abnormal thinking and behavior change, including “sleepdriving” and other complex behaviors while not being fully awake (preparing and eating food, making phone calls, or having sex). Tell patients to call you immediately if they develop any of these symptoms.
Severe Allergic Reactions
Inform patients that severe allergic reactions can occur from flurazepam. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if these occur.
Suicide
Tell patients that flurazepam can worsen depression, and to immediately report any suicidal thoughts.
Alcohol and Other Drugs
Ask patients about alcohol consumption, medicines they are taking now, and drugs they may be taking without a prescription. Advise patients that alcohol generally should not be used during treatment with flurazepam.
Pregnancy
Instruct patients to inform you if they are nursing or pregnant, or may become pregnant while taking flurazepam. If a woman becomes pregnant while taking flurazepam, she should discontinue use immediately.
Tolerance, Abuse, and Dependence
Tell patients not to increase the dose of flurazepam on their own, and to inform you if they believe the drug “does not work”. |alcohol=*Additive effects occur with concomitant use of alcohol.
- Sleep driving and other complex behaviors while not fully awake: Risk increases with dose and concomitant CNS depressants and alcohol.
|brandNames=*Dalmane[1] }} {{#subobject:
|Label Page=Flurazepam hydrochloride |Label Name=Flurazepam hydrochloride.png
}}
{{#subobject:
|Label Page=Flurazepam hydrochloride |Label Name=Flurazepam hydrochloride 30mg.png
}}