Familial hyperchylomicronemia: Difference between revisions
Aysha Aslam (talk | contribs) No edit summary |
|||
| (78 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}}{{VD}} | {{CMG}}{{AE}}{{VD}}{{AAA}} | ||
{{ | |||
'''To view Lipoprotein disorders main page [[ Lipoprotein disorders | Click here]]'''<br> | |||
'''To view Hyperlipoproteinemia main page [[ Hyperlipoproteinemia | Click here]]''' <br> | |||
{{SK}} Type I hyperlipoproteinemia, Burger-Grutz syndrome, Primary hyperlipoproteinemia, lipoprotein lipase deficiency, LPL deficiency, Idiopathic hyperlipemia, Essential hyperlipemia, Familial hyperlipemia, Lipase D deficiency, Hyperlipoproteinemia type IA, Familial chylomicronemia, Familial lipoprotein lipase deficiency, and Familial hyperchylomicronemia. | |||
==Overview== | ==Overview== | ||
This very rare | This very rare hyperlipidemia is due to a deficiency of [[lipoprotein lipase]] (LPL) or altered [[apolipoprotein C2]], resulting in elevated [[chylomicron]] which are the particles that transfer fatty acids from the [[digestive tract]] to the [[liver]]. Lipoprotein lipase is also responsible for the initial breakdown of endogenously made triacylglycerides in the form of very low density lipoprotein ([[VLDL]]). As such, one would expect a defect in LPL to also result in elevated VLDL. Its prevalence is one in 1,000,000 population. | ||
==Historical Perspective== | |||
*In 1932, Familial LPL deficency was first described by Burger and Grutz<ref name="national organization of rare disorders2"><nowiki>{{</nowiki>http://rarediseases.org/rare-diseases/familial-lipoprotein-lipase-deficiency<nowiki>}}/Accessed on 7 November,2016</nowiki></ref> | |||
*In 1967, Fredrickson using paper electrophosresis, classified lipoprotein disorder<ref name="pmid329619322">{{cite journal| author=Culliton BJ| title=Fredrickson's bitter end at Hughes. | journal=Science | year= 1987 | volume= 236 | issue= 4807 | pages= 1417-8 | pmid=3296193 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3296193 }}</ref> | |||
==Classification== | ==Classification== | ||
There is no established classification system for Type I hyperlipoproteinemia. However, based on the pathogenesis involved type 1 hyperlipoproteinemia can be classified into:<ref name="pmid23525082">{{cite journal| author=Brahm A, Hegele RA| title=Hypertriglyceridemia. | journal=Nutrients | year= 2013 | volume= 5 | issue= 3 | pages= 981-1001 | pmid=23525082 | doi=10.3390/nu5030981 | pmc=3705331 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23525082 }}</ref> | |||
=== | |||
=== | |||
Type 1a: Lipoprotein lipase deficiency | |||
Type 1b: Apolipoprotein c-IIdeficiency | |||
Type 1c: Presence of lipoprotein lipase inhibitor | |||
==Pathophysiology== | ==Pathophysiology== | ||
*Type I hyperlipoproteinemia is a rare autosomal recessive disorder of lipoprotein metabolism. <ref name=" | *Type I hyperlipoproteinemia is a rare [[autosomal recessive disorder]] of lipoprotein metabolism.<ref name="pmid275781122">{{cite journal| author=Pingitore P, Lepore SM, Pirazzi C, Mancina RM, Motta BM, Valenti L et al.| title=Identification and characterization of two novel mutations in the LPL gene causing type I hyperlipoproteinemia. | journal=J Clin Lipidol | year= 2016 | volume= 10 | issue= 4 | pages= 816-23 | pmid=27578112 | doi=10.1016/j.jacl.2016.02.015 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27578112 }}</ref><ref name="pmid234759572">{{cite journal| author=Young SG, Zechner R| title=Biochemistry and pathophysiology of intravascular and intracellular lipolysis. | journal=Genes Dev | year= 2013 | volume= 27 | issue= 5 | pages= 459-84 | pmid=23475957 | doi=10.1101/gad.209296.112 | pmc=3605461 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23475957 }}</ref><ref name="pmid151156922">{{cite journal| author=Pasalić D, Jurcić Z, Stipancić G, Ferencak G, Leren TP, Djurovic S et al.| title=Missense mutation W86R in exon 3 of the lipoprotein lipase gene in a boy with chylomicronemia. | journal=Clin Chim Acta | year= 2004 | volume= 343 | issue= 1-2 | pages= 179-84 | pmid=15115692 | doi=10.1016/j.cccn.2004.01.029 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15115692}}</ref><ref name="pmid11334614">{{cite journal| author=Gilbert B, Rouis M, Griglio S, de Lumley L, Laplaud P| title=Lipoprotein lipase (LPL) deficiency: a new patient homozygote for the preponderant mutation Gly188Glu in the human LPL gene and review of reported mutations: 75 % are clustered in exons 5 and 6. | journal=Ann Genet | year= 2001 | volume= 44 | issue= 1 | pages= 25-32 | pmid=11334614 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11334614 }}</ref> | ||
===Pathogenesis=== | ===Pathogenesis=== | ||
*Lipoprotein lipase(LPL) | *Lipoprotein lipase(LPL) hydrolyzes triglyceride-rich lipoproteins (TG) such as [[chylomicrons]] and very low-density lipoproteins. It catalyzes, the removal of [[Triglyceride|TG]] from the bloodstream generating free fatty acids for tissues. | ||
*For full enzymatic activity, LPL requires following cofactors: | *For full enzymatic activity, LPL requires following cofactors:<ref name="pmid275781122" /> | ||
**Apolipoprotein C-II and apolipoprotein A-V that are LPL activators | **[[Apolipoprotein C-II]] and apolipoprotein A-V that are LPL activators | ||
**Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein | **[[Glycosylphosphatidylinositol]]-anchored high-density lipoprotein-binding protein | ||
**Lipase maturation factor 1 | **[[Lipase maturation factor 1]] | ||
*Familial lipoprotein lipase inhibitor inherited in an [[autosomal dominant]] fashion. Inhibit the action of [[lipoprotein lipase]], resulting in decreased postheparin plasma LPL activity, elevated adipose tissue LPL activity, and normal plasma levels of functional [[apoC-I1]].<ref name=":02">https://ghr.nlm.nih.gov/gene/LPL#conditions</ref> | |||
*Functionally inactive or absent lipoprotein lipase enzyme, results in massive accumulation of chylomicrons, with extremely high level of plasma triglycerides. | |||
===Genetics=== | |||
*More than 220 mutations in the LPL gene have been found to cause familial lipoprotein lipase deficiency. The most common mutation in people of European ancestry replaces the protein building block (amino acid) glycine with the amino acid glutamic acid at position 188 in the enzyme (written as Gly188Glu or G188E).<ref name=":02" /> | |||
==Causes== | ==Causes== | ||
The cause of type 1 hyperlipidemia remains genetic. | The cause of type 1 hyperlipidemia remains genetic.<ref name="national organization of rare disorders2" /><ref name="rare diseasediorders2">https://rarediseases.info.nih.gov/diseases/6414/hyperlipoproteinemia-type-1 Accessed on 7 November,2016</ref><ref name="pmid275781122" /><ref name="pmid11893776">{{cite journal| author=Peterson J, Ayyobi AF, Ma Y, Henderson H, Reina M, Deeb SS et al.| title=Structural and functional consequences of missense mutations in exon 5 of the lipoprotein lipase gene. | journal=J Lipid Res | year= 2002 | volume= 43 | issue= 3 | pages= 398-406 | pmid=11893776 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11893776 }}</ref> | ||
{| class="wikitable" | |||
! colspan="1" rowspan="1" |Gene 1 | |||
! colspan="1" rowspan="1" |Variants Detected 2 | |||
! colspan="1" rowspan="1" |Variant Detection Frequency by Test Method 3 | |||
| colspan="1" rowspan="2" |''LPL'' | |||
| colspan="1" rowspan="1" |Sequence variants 4 (including p.Gly188Glu 5) | |||
| colspan="1" rowspan="1" |~97% 6 | |||
|- | |||
| colspan="1" rowspan="1" |Partial- and whole-gene deletions and duplications | |||
| colspan="1" rowspan="1" |~3% 8 | |||
|} | |||
==Differential diagnosis== | ==Differential diagnosis== | ||
{| class="wikitable" | |||
! | |||
|} | |||
==Epidemiology and Demographics== | ==Epidemiology and Demographics== | ||
Epidemiological and demographics of familial hyperchylomicronemia are discussed below:<ref name="pmid275781122" /><ref name="rare diseasediorders2" /><ref name="national organization of rare disorders2" /> | |||
Prevalence | ===Prevalence=== | ||
*The prevalence of familial LPL deficiency is approximately one in 1,000,000 in the general US population | |||
The prevalence of familial LPL deficiency is approximately one in 1,000,000 in the general US population | ===Demographics=== | ||
====Age==== | |||
*25% of affected children develop symptoms before one year of age. | |||
*The majority of patients develop symptoms before ten years of age. | |||
*Few individuals develop symptoms, at the time of pregnancy. | |||
====Gender==== | |||
== | *Males and females are equally affected by familial chylomicronemia.. | ||
====Race==== | |||
*The disease has been described in all races. | |||
*The prevalence is much higher in some areas of Quebec, Canada, as a result of a founder effect. | |||
==Screening== | ==Screening== | ||
*There are no screening guidelines for . | *There are no screening guidelines for Familial hyperchylomicronemia.<ref name="rare diseasediorders2" /><ref name="pmid275781122" /><ref name="medline plus2">https://medlineplus.gov/ency/article/000405.htm</ref> | ||
* | *It may be appropriate to measure plasma triglyceride concentration in at-risk siblings during infancy; early diagnosis and implementation of dietary fat intake restriction can prevent symptoms and related medical complications. | ||
==Natural History, Complications, and Prognosis== | |||
Natural history, complications and prognosis of type 1 hyperlipoproteinemia include:<ref name="medline plus2" /><ref name="pmid12387965" /><ref name="pmid9738727" /> | |||
===Natural History=== | ===Natural History=== | ||
If left untreated, pancreatitis can develop into a chronic condition that can damage the pancreas and, in rare cases | *If left untreated, [[pancreatitis]] can develop into a chronic condition that can damage the pancreas and, in rare cases it could be life-threatening. | ||
===Complications=== | |||
=== | *Pancreatitis and recurrent episodes of abdominal pain may develop. | ||
Pancreatitis and recurrent episodes of abdominal pain may develop. | *Intestinal ischemia | ||
*Depression, | |||
*Memory loss | |||
*Intellectual decline | |||
===Prognosis=== | ===Prognosis=== | ||
*People with this condition who follow a very low-fat diet | *People with this condition who follow a very low-fat diet have a good prognosis. | ||
==Diagnosis== | ==Diagnosis== | ||
===History and symptoms=== | |||
Presumptive diagnosis can be made, when an infant presents with a history of failure to thrive or recurrent abdominal pain with a documented high fasting plasma triglyceride concentration.<ref name="GeneReviews2"><nowiki>{{</nowiki>https://www.ncbi.nlm.nih.gov/books/NBK1308/<nowiki>}} Accessed on 7 November,2016</nowiki></ref><ref name="medline plus2" /> | |||
Symptoms of Type I hyperlipoproteinemia include:<ref name="rare diseasediorders2" /><ref name="medline plus2" /><ref name="pmid232573032">{{cite journal| author=Robinson JG| title=What is the role of advanced lipoprotein analysis in practice? | journal=J Am Coll Cardiol | year= 2012 | volume= 60 | issue= 25 | pages= 2607-15 | pmid=23257303 | doi=10.1016/j.jacc.2012.04.067 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23257303 }}</ref><ref name="pmid9738727">{{cite journal| author=Feoli-Fonseca JC, Lévy E, Godard M, Lambert M| title=Familial lipoprotein lipase deficiency in infancy: clinical, biochemical, and molecular study. | journal=J Pediatr | year= 1998 | volume= 133 | issue= 3 | pages= 417-23 | pmid=9738727 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9738727 }}</ref> | |||
*Abdominal pain (may appear as colic in infancy) | |||
*Loss of appetite, fatigue and irritability | |||
*Nausea | |||
*Pain in the muscles and bones (musculoskeletal pain) | |||
*Vomiting | |||
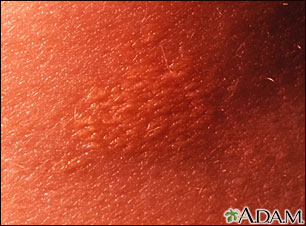
*Small yellow papules localized over the trunk, buttocks, knees, and extensor surfaces of the arms | |||
*Blood in stools(Intestinal ischemia) | |||
*In rare cases, neurological features of depression, memory loss, and mild intellectual decline (dementia) develop | |||
===Physical examination=== | |||
Signs of Type 1 hyperlipoproteinemia include:<ref name="rare diseasediorders2" /><ref name="medline plus2" /><ref name="pmid232573032" /><ref name="pmid9738727" /><ref name="pmid12387965" /> | |||
* Enlarged liver and spleen | |||
* Failure to thrive in infancy | |||
* Fatty deposits in the skin (xanthomas) | |||
* Pale retinas and white-colored blood vessels in the retinas | |||
* Pancreatitis that keeps returning | |||
* Yellowing of the eyes and skin (jaundice) | |||
<gallery> | |||
File:Xanthoma-close-up.jpg | |||
</gallery> | |||
===Laboratory findings=== | |||
*Diagnosis of Type I hyperlipoproteinemia is confirmed by detection of low or absent LPL enzyme activity in an assay system, that contains either normal plasma or apoprotein C-II excluding hepatic lipase. | |||
*Laboratory findings consistent with the Type I hyperlipoproteinemia include the following:<ref name="national organization of rare disorders2" /><ref name="rare diseasediorders2" /><ref name="pmid232573032" /> | |||
{| class="wikitable" | {| class="wikitable" | ||
! colspan=" | ! colspan="11" |Laboratory finding | ||
|- | |- | ||
|Phenotype | |Phenotype | ||
| Line 171: | Line 117: | ||
|Serum total | |Serum total | ||
cholesterol | cholesterol | ||
|Serum | |HDL | ||
|VLDL | |||
|Serum | |||
triglycerides | triglycerides | ||
|Plasma | |Plasma | ||
appearance | appearance | ||
|Postheparin | |Postheparin | ||
lipolytic | lipolytic | ||
activity | activity | ||
|Glucose | |Glucose | ||
tolerance | tolerance | ||
|Carbohydrate | |Carbohydrate | ||
| Line 186: | Line 134: | ||
|- | |- | ||
|Hyperlipoproteinemia type 1 | |Hyperlipoproteinemia type 1 | ||
|Chylomicrons | |Chylomicrons '''↑↑↑↑''' | ||
|Normal to | |Normal to | ||
elevated | elevated | ||
| | |'''↓↓↓''' | ||
|'''↓''' | |||
|'''↑↑↑↑''' | |||
|Creamy | |Creamy | ||
|Decreased | |Decreased | ||
|Normal | |Normal | ||
|May be abnormal | |May be abnormal | ||
|Markedly abnormal | |Markedly abnormal | ||
|} | |} | ||
===Molecular Genetic Testing=== | |||
Testing | *Diagnosis can be confirmed by molecular genetic testing that can detect mutations in the LPL gene.<ref name="pmid275781122" /> | ||
*The test is often not necessary to confirm a diagnosis of type I hyperlipidemia. | |||
The | |||
==Treatment== | ==Treatment== | ||
Treatment for hyperlipoproteinemia type 1 is intended to control blood triglyceride levels. There is currently no pharmacotherapy approved for the treatment of familial hyperchylomicronemia in the United States The mainstay of treatment includes dietary modification and control. | |||
===Medical Therapy=== | |||
Pregnancy Management | There is currently no pharmacotherapy approved for the treatment of familial hyperchylomicronemia in the United States | ||
===Dietary Management=== | |||
Dietary management of hyperlipoproteinemia type 1 include the following:<ref name="rare diseasediorders2" /><ref name="GeneReviews2" /> <ref name="medline plus2" /> | |||
*Controlling blood triglyceride levels with a very low-fat diet | |||
*It is recommended that individuals with this condition eat no more than 20 grams of fat per day. | |||
*Medium-chain fatty acids (such as coconut oil) can be incorporated into the diet, as they are absorbed by the body in a different manner. | |||
*Dietary counseling may be helpful to maintain adequate calorie and nutrient intake. | |||
===Pregnancy Management=== | |||
*Pregnant women may experience significant changes in lipid level in second and third trimester, and may require strategies to lower fat intake. Pregnancy management of type 1hyperlipoproteinemia involves the following:<ref name="pmid119833472">{{cite journal| author=Al-Shali K, Wang J, Fellows F, Huff MW, Wolfe BM, Hegele RA| title=Successful pregnancy outcome in a patient with severe chylomicronemia due to compound heterozygosity for mutant lipoprotein lipase. | journal=Clin Biochem | year= 2002 | volume= 35 | issue= 2 | pages= 125-30 | pmid=11983347 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11983347 }}</ref> | |||
*Periodic assessment of plasma triglycerides is highly recommended | |||
*Comprehensive analysis of risks versus benefits is required before the use of fibrates, nicotinic acid and omega-3 fatty acid | |||
===Investigative Therapies=== | |||
*Orlistat, in conjunction with a low fat diet has been used to treat some patients with familial hyperchilomicronemia caused by compound heterozygous LPL deficiency.<ref name="pmid234154322">{{cite journal| author=Blackett P, Tryggestad J, Krishnan S, Li S, Xu W, Alaupovic P et al.| title=Lipoprotein abnormalities in compound heterozygous lipoprotein lipase deficiency after treatment with a low-fat diet and orlistat. | journal=J Clin Lipidol | year= 2013 | volume= 7 | issue= 2 | pages= 132-9 | pmid=23415432 | doi=10.1016/j.jacl.2012.11.006 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23415432 }}</ref> | |||
===Gene Therapy=== | |||
*Alipogene tipavovec(Glybera) gene therapy was approved by European commission(2012), in treating adult patients with recurrent episodes of pancreatitis.<ref name="pmid274124552">{{cite journal| author=Gaudet D, Stroes ES, Méthot J, Brisson D, Tremblay K, Bernelot Moens SJ et al.| title=Long-Term Retrospective Analysis of Gene Therapy with Alipogene Tiparvovec and Its Effect on Lipoprotein Lipase Deficiency-Induced Pancreatitis. | journal=Hum Gene Ther | year= 2016 | volume= | issue= | pages= | pmid=27412455 | doi=10.1089/hum.2015.158 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27412455 }}</ref> | |||
==Prevention== | ==Prevention== | ||
Genetic counseling | === Primary Prevention === | ||
*There are no primary preventive measures to protect against type I hyperlipoproteinemia. There is currently no known intervention to prevent someone from inheriting this condition.<ref name="rare diseasediorders2" /> | |||
*Genetic counseling is recommended for patients and family members.<ref name="pmid277719612">{{cite journal| author=Ahmad Z, Halter R, Stevenson M| title=Building a better understanding of the burden of disease in familial chylomicronemia syndrome. | journal=Expert Rev Clin Pharmacol | year= 2016 | volume= | issue= | pages= 1-3 | pmid=27771961 | doi=10.1080/17512433.2017.1251839 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27771961 }}</ref> | |||
===Secondary prevention=== | |||
Prevention of | Secondary prevention involves the following:<ref name="medline plus2" /><ref name="pmid27771961">{{cite journal| author=Ahmad Z, Halter R, Stevenson M| title=Building a better understanding of the burden of disease in familial chylomicronemia syndrome. | journal=Expert Rev Clin Pharmacol | year= 2016 | volume= | issue= | pages= 1-3 | pmid=27771961 | doi=10.1080/17512433.2017.1251839 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27771961 }}</ref> | ||
*Prevention of acute recurrent pancreatitis decreases the risk of development of diabetes mellitus and fat malabsorption. | |||
*Maintaining the plasma triglyceride concentration at less than 2000 mg/dl, keeps the individual with familial LPL deficiency free of symptoms. This can be accomplished by restriction of dietary fat to no more than 20 g/day or 15% of total energy intake. | |||
*Periodic assessment of plasma triglycerides levels is highly recommended. | |||
*Patients should avoid agents that increased endogenous triglyceride levels like alcohol, diuretics, oral estrogens, isoretinoin, glucocorticords, and beta-blockers. | |||
==References== | |||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Lipopedia]] | [[Category:Lipopedia]] | ||
[[Category:Cardiology]] | |||
{{Lipopedia}} | {{Lipopedia}} | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Latest revision as of 02:34, 18 July 2021
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]Associate Editor(s)-in-Chief: Vishal Devarkonda, M.B.B.S[2]Aysha Aslam, M.B.B.S[3]
To view Lipoprotein disorders main page Click here
To view Hyperlipoproteinemia main page Click here
Synonyms and keywords: Type I hyperlipoproteinemia, Burger-Grutz syndrome, Primary hyperlipoproteinemia, lipoprotein lipase deficiency, LPL deficiency, Idiopathic hyperlipemia, Essential hyperlipemia, Familial hyperlipemia, Lipase D deficiency, Hyperlipoproteinemia type IA, Familial chylomicronemia, Familial lipoprotein lipase deficiency, and Familial hyperchylomicronemia.
Overview
This very rare hyperlipidemia is due to a deficiency of lipoprotein lipase (LPL) or altered apolipoprotein C2, resulting in elevated chylomicron which are the particles that transfer fatty acids from the digestive tract to the liver. Lipoprotein lipase is also responsible for the initial breakdown of endogenously made triacylglycerides in the form of very low density lipoprotein (VLDL). As such, one would expect a defect in LPL to also result in elevated VLDL. Its prevalence is one in 1,000,000 population.
Historical Perspective
- In 1932, Familial LPL deficency was first described by Burger and Grutz[1]
- In 1967, Fredrickson using paper electrophosresis, classified lipoprotein disorder[2]
Classification
There is no established classification system for Type I hyperlipoproteinemia. However, based on the pathogenesis involved type 1 hyperlipoproteinemia can be classified into:[3]
Type 1a: Lipoprotein lipase deficiency
Type 1b: Apolipoprotein c-IIdeficiency
Type 1c: Presence of lipoprotein lipase inhibitor
Pathophysiology
- Type I hyperlipoproteinemia is a rare autosomal recessive disorder of lipoprotein metabolism.[4][5][6][7]
Pathogenesis
- Lipoprotein lipase(LPL) hydrolyzes triglyceride-rich lipoproteins (TG) such as chylomicrons and very low-density lipoproteins. It catalyzes, the removal of TG from the bloodstream generating free fatty acids for tissues.
- For full enzymatic activity, LPL requires following cofactors:[4]
- Apolipoprotein C-II and apolipoprotein A-V that are LPL activators
- Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein
- Lipase maturation factor 1
- Familial lipoprotein lipase inhibitor inherited in an autosomal dominant fashion. Inhibit the action of lipoprotein lipase, resulting in decreased postheparin plasma LPL activity, elevated adipose tissue LPL activity, and normal plasma levels of functional apoC-I1.[8]
- Functionally inactive or absent lipoprotein lipase enzyme, results in massive accumulation of chylomicrons, with extremely high level of plasma triglycerides.
Genetics
- More than 220 mutations in the LPL gene have been found to cause familial lipoprotein lipase deficiency. The most common mutation in people of European ancestry replaces the protein building block (amino acid) glycine with the amino acid glutamic acid at position 188 in the enzyme (written as Gly188Glu or G188E).[8]
Causes
The cause of type 1 hyperlipidemia remains genetic.[1][9][4][10]
| Gene 1 | Variants Detected 2 | Variant Detection Frequency by Test Method 3 | LPL | Sequence variants 4 (including p.Gly188Glu 5) | ~97% 6 |
|---|---|---|---|---|---|
| Partial- and whole-gene deletions and duplications | ~3% 8 |
Differential diagnosis
Epidemiology and Demographics
Epidemiological and demographics of familial hyperchylomicronemia are discussed below:[4][9][1]
Prevalence
- The prevalence of familial LPL deficiency is approximately one in 1,000,000 in the general US population
Demographics
Age
- 25% of affected children develop symptoms before one year of age.
- The majority of patients develop symptoms before ten years of age.
- Few individuals develop symptoms, at the time of pregnancy.
Gender
- Males and females are equally affected by familial chylomicronemia..
Race
- The disease has been described in all races.
- The prevalence is much higher in some areas of Quebec, Canada, as a result of a founder effect.
Screening
- There are no screening guidelines for Familial hyperchylomicronemia.[9][4][11]
- It may be appropriate to measure plasma triglyceride concentration in at-risk siblings during infancy; early diagnosis and implementation of dietary fat intake restriction can prevent symptoms and related medical complications.
Natural History, Complications, and Prognosis
Natural history, complications and prognosis of type 1 hyperlipoproteinemia include:[11][12][13]
Natural History
- If left untreated, pancreatitis can develop into a chronic condition that can damage the pancreas and, in rare cases it could be life-threatening.
Complications
- Pancreatitis and recurrent episodes of abdominal pain may develop.
- Intestinal ischemia
- Depression,
- Memory loss
- Intellectual decline
Prognosis
- People with this condition who follow a very low-fat diet have a good prognosis.
Diagnosis
History and symptoms
Presumptive diagnosis can be made, when an infant presents with a history of failure to thrive or recurrent abdominal pain with a documented high fasting plasma triglyceride concentration.[14][11]
Symptoms of Type I hyperlipoproteinemia include:[9][11][15][13]
- Abdominal pain (may appear as colic in infancy)
- Loss of appetite, fatigue and irritability
- Nausea
- Pain in the muscles and bones (musculoskeletal pain)
- Vomiting
- Small yellow papules localized over the trunk, buttocks, knees, and extensor surfaces of the arms
- Blood in stools(Intestinal ischemia)
- In rare cases, neurological features of depression, memory loss, and mild intellectual decline (dementia) develop
Physical examination
Signs of Type 1 hyperlipoproteinemia include:[9][11][15][13][12]
- Enlarged liver and spleen
- Failure to thrive in infancy
- Fatty deposits in the skin (xanthomas)
- Pale retinas and white-colored blood vessels in the retinas
- Pancreatitis that keeps returning
- Yellowing of the eyes and skin (jaundice)
Laboratory findings
- Diagnosis of Type I hyperlipoproteinemia is confirmed by detection of low or absent LPL enzyme activity in an assay system, that contains either normal plasma or apoprotein C-II excluding hepatic lipase.
- Laboratory findings consistent with the Type I hyperlipoproteinemia include the following:[1][9][15]
| Laboratory finding | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phenotype | Lipoprotein(s)
Elevated |
Serum total
cholesterol |
HDL | VLDL | Serum
triglycerides |
Plasma
appearance |
Postheparin
lipolytic activity |
Glucose
tolerance |
Carbohydrate
inducibility |
Fat tolerance |
| Hyperlipoproteinemia type 1 | Chylomicrons ↑↑↑↑ | Normal to
elevated |
↓↓↓ | ↓ | ↑↑↑↑ | Creamy | Decreased | Normal | May be abnormal | Markedly abnormal |
Molecular Genetic Testing
- Diagnosis can be confirmed by molecular genetic testing that can detect mutations in the LPL gene.[4]
- The test is often not necessary to confirm a diagnosis of type I hyperlipidemia.
Treatment
Treatment for hyperlipoproteinemia type 1 is intended to control blood triglyceride levels. There is currently no pharmacotherapy approved for the treatment of familial hyperchylomicronemia in the United States The mainstay of treatment includes dietary modification and control.
Medical Therapy
There is currently no pharmacotherapy approved for the treatment of familial hyperchylomicronemia in the United States
Dietary Management
Dietary management of hyperlipoproteinemia type 1 include the following:[9][14] [11]
- Controlling blood triglyceride levels with a very low-fat diet
- It is recommended that individuals with this condition eat no more than 20 grams of fat per day.
- Medium-chain fatty acids (such as coconut oil) can be incorporated into the diet, as they are absorbed by the body in a different manner.
- Dietary counseling may be helpful to maintain adequate calorie and nutrient intake.
Pregnancy Management
- Pregnant women may experience significant changes in lipid level in second and third trimester, and may require strategies to lower fat intake. Pregnancy management of type 1hyperlipoproteinemia involves the following:[16]
- Periodic assessment of plasma triglycerides is highly recommended
- Comprehensive analysis of risks versus benefits is required before the use of fibrates, nicotinic acid and omega-3 fatty acid
Investigative Therapies
- Orlistat, in conjunction with a low fat diet has been used to treat some patients with familial hyperchilomicronemia caused by compound heterozygous LPL deficiency.[17]
Gene Therapy
- Alipogene tipavovec(Glybera) gene therapy was approved by European commission(2012), in treating adult patients with recurrent episodes of pancreatitis.[18]
Prevention
Primary Prevention
- There are no primary preventive measures to protect against type I hyperlipoproteinemia. There is currently no known intervention to prevent someone from inheriting this condition.[9]
- Genetic counseling is recommended for patients and family members.[19]
Secondary prevention
Secondary prevention involves the following:[11][20]
- Prevention of acute recurrent pancreatitis decreases the risk of development of diabetes mellitus and fat malabsorption.
- Maintaining the plasma triglyceride concentration at less than 2000 mg/dl, keeps the individual with familial LPL deficiency free of symptoms. This can be accomplished by restriction of dietary fat to no more than 20 g/day or 15% of total energy intake.
- Periodic assessment of plasma triglycerides levels is highly recommended.
- Patients should avoid agents that increased endogenous triglyceride levels like alcohol, diuretics, oral estrogens, isoretinoin, glucocorticords, and beta-blockers.
References
- ↑ 1.0 1.1 1.2 1.3 {{http://rarediseases.org/rare-diseases/familial-lipoprotein-lipase-deficiency}}/Accessed on 7 November,2016
- ↑ Culliton BJ (1987). "Fredrickson's bitter end at Hughes". Science. 236 (4807): 1417–8. PMID 3296193.
- ↑ Brahm A, Hegele RA (2013). "Hypertriglyceridemia". Nutrients. 5 (3): 981–1001. doi:10.3390/nu5030981. PMC 3705331. PMID 23525082.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Pingitore P, Lepore SM, Pirazzi C, Mancina RM, Motta BM, Valenti L; et al. (2016). "Identification and characterization of two novel mutations in the LPL gene causing type I hyperlipoproteinemia". J Clin Lipidol. 10 (4): 816–23. doi:10.1016/j.jacl.2016.02.015. PMID 27578112.
- ↑ Young SG, Zechner R (2013). "Biochemistry and pathophysiology of intravascular and intracellular lipolysis". Genes Dev. 27 (5): 459–84. doi:10.1101/gad.209296.112. PMC 3605461. PMID 23475957.
- ↑ Pasalić D, Jurcić Z, Stipancić G, Ferencak G, Leren TP, Djurovic S; et al. (2004). "Missense mutation W86R in exon 3 of the lipoprotein lipase gene in a boy with chylomicronemia". Clin Chim Acta. 343 (1–2): 179–84. doi:10.1016/j.cccn.2004.01.029. PMID 15115692.
- ↑ Gilbert B, Rouis M, Griglio S, de Lumley L, Laplaud P (2001). "Lipoprotein lipase (LPL) deficiency: a new patient homozygote for the preponderant mutation Gly188Glu in the human LPL gene and review of reported mutations: 75 % are clustered in exons 5 and 6". Ann Genet. 44 (1): 25–32. PMID 11334614.
- ↑ 8.0 8.1 https://ghr.nlm.nih.gov/gene/LPL#conditions
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 https://rarediseases.info.nih.gov/diseases/6414/hyperlipoproteinemia-type-1 Accessed on 7 November,2016
- ↑ Peterson J, Ayyobi AF, Ma Y, Henderson H, Reina M, Deeb SS; et al. (2002). "Structural and functional consequences of missense mutations in exon 5 of the lipoprotein lipase gene". J Lipid Res. 43 (3): 398–406. PMID 11893776.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 https://medlineplus.gov/ency/article/000405.htm
- ↑ 12.0 12.1
- ↑ 13.0 13.1 13.2 Feoli-Fonseca JC, Lévy E, Godard M, Lambert M (1998). "Familial lipoprotein lipase deficiency in infancy: clinical, biochemical, and molecular study". J Pediatr. 133 (3): 417–23. PMID 9738727.
- ↑ 14.0 14.1 {{https://www.ncbi.nlm.nih.gov/books/NBK1308/}} Accessed on 7 November,2016
- ↑ 15.0 15.1 15.2 Robinson JG (2012). "What is the role of advanced lipoprotein analysis in practice?". J Am Coll Cardiol. 60 (25): 2607–15. doi:10.1016/j.jacc.2012.04.067. PMID 23257303.
- ↑ Al-Shali K, Wang J, Fellows F, Huff MW, Wolfe BM, Hegele RA (2002). "Successful pregnancy outcome in a patient with severe chylomicronemia due to compound heterozygosity for mutant lipoprotein lipase". Clin Biochem. 35 (2): 125–30. PMID 11983347.
- ↑ Blackett P, Tryggestad J, Krishnan S, Li S, Xu W, Alaupovic P; et al. (2013). "Lipoprotein abnormalities in compound heterozygous lipoprotein lipase deficiency after treatment with a low-fat diet and orlistat". J Clin Lipidol. 7 (2): 132–9. doi:10.1016/j.jacl.2012.11.006. PMID 23415432.
- ↑ Gaudet D, Stroes ES, Méthot J, Brisson D, Tremblay K, Bernelot Moens SJ; et al. (2016). "Long-Term Retrospective Analysis of Gene Therapy with Alipogene Tiparvovec and Its Effect on Lipoprotein Lipase Deficiency-Induced Pancreatitis". Hum Gene Ther. doi:10.1089/hum.2015.158. PMID 27412455.
- ↑ Ahmad Z, Halter R, Stevenson M (2016). "Building a better understanding of the burden of disease in familial chylomicronemia syndrome". Expert Rev Clin Pharmacol: 1–3. doi:10.1080/17512433.2017.1251839. PMID 27771961.
- ↑ Ahmad Z, Halter R, Stevenson M (2016). "Building a better understanding of the burden of disease in familial chylomicronemia syndrome". Expert Rev Clin Pharmacol: 1–3. doi:10.1080/17512433.2017.1251839. PMID 27771961.