Eprosartan: Difference between revisions
Joao Silva (talk | contribs) No edit summary |
Joao Silva (talk | contribs) No edit summary |
||
| Line 172: | Line 172: | ||
: (list/description of adverse reactions) | : (list/description of adverse reactions) | ||
|postmarketing=(Description) | |postmarketing=(Description) | ||
|drugInteractions=* | |drugInteractions=* Digoxin - concomitant administration of eprosartan and [[digoxin]] had no effect on single oral-dose [[digoxin]] [[pharmacokinetics]]. | ||
* | * Warfarin - concomitant administration of eprosartan and [[warfarin]] had no effect on steady-state [[prothrombin time]] ratios (INR) in healthy volunteers. | ||
* | * Glyburide - concomitant administration of eprosartan and [[glyburide]] in [[diabetic]] patients did not affect 24-hour [[plasma glucose]] profiles. | ||
* | * Ranitidine - eprosartan [[pharmacokinetics]] were not affected by concomitant administration of [[ranitidine]]. | ||
* Eprosartan did not inhibit human [[cytochrome P450]] [[enzymes]] CYP1A, 2A6, 2C9/8, 2C19, 2D6, 2E and 3A in vitro. | * Eprosartan did not inhibit human [[cytochrome P450]] [[enzymes]] CYP1A, 2A6, 2C9/8, 2C19, 2D6, 2E and 3A in vitro. | ||
* Eprosartan is not metabolized by the [[cytochrome P450]] system. | * Eprosartan is not metabolized by the [[cytochrome P450]] system. | ||
* Eprosartan steady-state [[concentrations]] were not affected by concomitant administration of [[ketoconazole]] or [[fluconazole]], potent inhibitors of CYP3A and 2C9, respectively. | * Ketoconazole and fluconazole - Eprosartan steady-state [[concentrations]] were not affected by concomitant administration of [[ketoconazole]] or [[fluconazole]], potent inhibitors of CYP3A and 2C9, respectively. | ||
|useInPregnancyFDA=(Description) | |useInPregnancyFDA=(Description) | ||
|useInPregnancyAUS=(Description) | |useInPregnancyAUS=(Description) | ||
Revision as of 22:05, 1 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
Condition Name:
See full prescribing information for complete boxed warning.
|
Overview
Eprosartan is an angiotensin II receptor blocker that is FDA approved for the {{{indicationType}}} of hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include abdominal pain, myalgia, dizziness, upper respiratory infection and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
Condition Name:
See full prescribing information for complete boxed warning.
|
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Digoxin - concomitant administration of eprosartan and digoxin had no effect on single oral-dose digoxin pharmacokinetics.
- Warfarin - concomitant administration of eprosartan and warfarin had no effect on steady-state prothrombin time ratios (INR) in healthy volunteers.
- Glyburide - concomitant administration of eprosartan and glyburide in diabetic patients did not affect 24-hour plasma glucose profiles.
- Ranitidine - eprosartan pharmacokinetics were not affected by concomitant administration of ranitidine.
- Eprosartan did not inhibit human cytochrome P450 enzymes CYP1A, 2A6, 2C9/8, 2C19, 2D6, 2E and 3A in vitro.
- Eprosartan is not metabolized by the cytochrome P450 system.
- Ketoconazole and fluconazole - Eprosartan steady-state concentrations were not affected by concomitant administration of ketoconazole or fluconazole, potent inhibitors of CYP3A and 2C9, respectively.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
- Eprosartan pharmacokinetics have not been investigated in patients younger than 18 years of age.
Geriatic Use
- Following single oral dose administration of eprosartan to healthy elderly men (aged 68 to 78 years), AUC, Cmax, and Tmax eprosartan values increased, on average by approximately twofold, compared to healthy young men (aged 20 to 39 years) who received the same dose.
- The extent of plasma protein binding was not influenced by age.
Gender
- There was no difference in the pharmacokinetics and plasma protein binding between men and women following single oral dose administration of eprosartan.
Race
- A pooled population pharmacokinetic analysis of 442 Caucasian and 29 non-Caucasian hypertensive patients showed that oral clearance and steady-state volume of distribution were not influenced by race.
Renal Impairment
- Following administration of 600 mg once daily, there was a 70-90% increase in AUC, and a 30-50% increase in Cmax in moderate or severe renal impairment. The unbound eprosartan fractions increased by 35% and 59% in patients with moderate and severe renal impairment, respectively. No initial dosing adjustment is generally necessary in patients with moderate or severe renal impairment, with maximum dose not exceeding 600 mg daily.
- Eprosartan was poorly removed by hemodialysis (CLHD<1 L/hr).
Hepatic Impairment
- Eprosartan AUC (but not Cmax) values increased, on average, by approximately 40% in men with decreased hepatic function compared to healthy men after a single 100 mg oral dose of eprosartan. Hepatic disease was defined as a documented clinical history of chronic hepatic abnormality diagnosed by liver biopsy, liver/spleen scan or clinical laboratory tests.
- The extent of eprosartan plasma protein binding was not influenced by hepatic dysfunction.
- No dosage adjustment is necessary for patients with hepatic impairment.
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Eprosartan
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Angiotensin II (formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme [kininase II]), a potent vasoconstrictor, is the principal pressor agent of the renin-angiotensin system. Angiotensin II also stimulates aldosterone synthesis and secretion by the adrenal cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth.
- Eprosartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor found in many tissues (e.g., vascular smooth muscle, adrenal gland). There is also an AT2 receptor found in many tissues but it is not known to be associated with cardiovascular homeostasis.
- Eprosartan does not exhibit any partial agonist activity at the AT1 receptor. Its affinity for the AT1 receptor is 1,000 times greater than for the AT2 receptor. In vitro binding studies indicate that eprosartan is a reversible, competitive inhibitor of the AT1 receptor.
- Blockade of the AT1 receptor removes the negative feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II do not overcome the effect of eprosartan on blood pressure.
- Eprosartan mesylate does not inhibit kininase II, the enzyme that converts angiotensin I to angiotensin II and degrades bradykinin; whether this has clinical relevance is not known. It does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Structure
- Eprosartan mesylate is a non-biphenyl non-tetrazole angiotensin II receptor (AT1) antagonist. A selective non-peptide molecule, eprosartan mesylate is chemically described as the monomethanesulfonate of (E )-2-butyl-1-(p-carboxybenzyl)-α-2-thienylmethylimid-azole-5-acrylic acid.
- Its empirical formula is C23H24N2O4S•CH4O3S and molecular weight is 520.625. Its structural formula is:
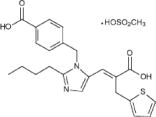
- Eprosartan mesylate is a white to off-white free-flowing crystalline powder that is insoluble in water, freely soluble in ethanol, and melts between 248°C and 250°C.
- Eprosartan mesylate is available as aqueous film-coated tablets containing eprosartan mesylate equivalent to 400 mg or 600 mg eprosartan zwitterion (pink, oval, non-scored tablets or white, non-scored, capsule-shaped tablets, respectively).
- Inactive Ingredients:
- The 400 mg tablet contains the following: croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide. The 600 mg tablet contains crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide.
Pharmacodynamics
- Eprosartan inhibits the pharmacologic effects of angiotensin II infusions in healthy adult men. Single oral doses of eprosartan from 10 mg to 400 mg have been shown to inhibit the vasopressor, renal vasoconstrictive and aldosterone secretory effects of infused angiotensin II with complete inhibition evident at doses of 350 mg and above. Eprosartan inhibits the pressor effects of angiotensin II infusions. A single oral dose of 350 mg of eprosartan inhibits pressor effects by approximately 100% at peak, with approximately 30% inhibition persisting for 24 hours. The absence of angiotensin II AT1 agonist activity has been demonstrated in healthy adult men. In hypertensive patients treated chronically with eprosartan, there was a twofold rise in angiotensin II plasma concentration and a twofold rise in plasma renin activity, while plasma aldosterone levels remained unchanged. Serum potassium levels also remained unchanged in these patients.
- Achievement of maximal blood pressure response to a given dose in most patients may take 2 to 3 weeks of treatment. Onset of blood pressure reduction is seen within 1 to 2 hours of dosing with few instances of orthostatic hypotension. Blood pressure control is maintained with once- or twice-daily dosing over a 24-hour period. Discontinuing treatment with eprosartan does not lead to a rapid rebound increase in blood pressure.
- There was no change in mean heart rate in patients treated with eprosartan in controlled clinical trials.
- Eprosartan increases mean effective renal plasma flow (ERPF) in salt-replete and salt-restricted normal subjects. A dose-related increase in ERPF of 25% to 30% occurred in salt-restricted normal subjects, with the effect plateauing between the 200 mg and 400 mg doses. There was no change in ERPF in hypertensive patients and patients with renal insufficiency on normal salt diets. Eprosartan did not reduce glomerular filtration rate in patients with renal insufficiency or in patients hypertension, after 7 days and 28 days of dosing, respectively. In hypertensive patients and patients with chronic renal insufficiency, eprosartan did not change fractional excretion of sodium and potassium.
- Eprosartan (1200 mg once daily for 7 days or 300 mg twice daily for 28 days) had no effect on the excretion of uric acid in healthy men, patients with essential hypertension or those with varying degrees of renal insufficiency.
- There were no effects on mean levels of fasting triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol or fasting glucose.
Pharmacokinetics
- General:
- Absolute bioavailability following a single 300 mg oral dose of eprosartan is approximately 13%.
- Eprosartan plasma concentrations peak at 1 to 2 hours after an oral dose in the fasted state.
- Administering eprosartan with food delays absorption, and causes variable changes (<25%) in Cmax and AUC values which do not appear clinically important. Plasma concentrations of eprosartan increase in a slightly less than dose-proportional manner over the 100 mg to 800 mg dose range. The mean terminal elimination half-life of eprosartan following multiple oral doses of 600 mg was approximately 20 hours.
- Eprosartan does not significantly accumulate with chronic use.
- Metabolism and Excretion:
- Eprosartan is eliminated by biliary and renal excretion, primarily as unchanged compound.
- Less than 2% of an oral dose is excreted in the urine as a glucuronide.
- There are no active metabolites following oral and intravenous dosing with [14C] eprosartan in human subjects.
- Eprosartan was the only drug-related compound found in the plasma and feces. Following intravenous [14C] eprosartan, about 61% of the material is recovered in the feces and about 37% in the urine. Following an oral dose of [14C] eprosartan, about 90% is recovered in the feces and about 7% in the urine. Approximately 20% of the radioactivity excreted in the urine was an acyl glucuronide of eprosartan with the remaining 80% being unchanged eprosartan.
- Distribution:
- Plasma protein binding of eprosartan is high (approximately 98%) and constant over the concentration range achieved with therapeutic doses.
- The pooled population pharmacokinetic analysis from two Phase 3 trials of 299 men and 172 women with mild to moderate hypertension (aged 20 to 93 years) showed that eprosartan exhibited a population mean oral clearance (CL/F) for an average 60-year-old patient of 48.5 L/hr. The population mean steady-state volume of distribution (Vss/F) was 308 L. Eprosartan pharmacokinetics were not influenced by weight, race, gender or severity of hypertension at baseline. Oral clearance was shown to be a linear function of age with CL/F decreasing 0.62 L/hr for every year increase.
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
- TEVETEN® is available as aqueous film-coated tablets as follows:
- 400 mg pink, non-scored, oval tablets, debossed with “5044” on one side.
- NDC 0074–3025–11 (bottles of 100)
- 600 mg white, non-scored, capsule-shaped tablets, debossed with “5046” on one side.
- NDC 0074–3040–11 (bottles of 100)
Storage
Store at controlled room temperature 20 to 25°C (68 to 77°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Eprosartan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eprosartan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
- Some patients previously exposed to eprosartan showed signs of alcohol intolerance, however, it is not possible to confirm if eprosartan was directly involved in the adverse reaction.
Brand Names
Teveten®
Look-Alike Drug Names
N/A
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.