Eculizumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
See full prescribing information for complete Boxed Warning.
Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early.
|
Overview
Eculizumab is a monoclonal antibody that is FDA approved for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis and for patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, diarrhea, nausea, vomiting, anemia, backache, headache, insomnia, nasal congestion, nasopharyngitis, respiratory tract infection and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Paroxysmal Nocturnal Hemoglobinuria (PNH)
Eculizumab is indicated for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis. Dosage:
- 600 mg weekly for the first 4 weeks, followed by
- 900 mg for the fifth dose 1 week later, then
- 900 mg every 2 weeks thereafter.
Atypical Hemolytic Uremic Syndrome (aHUS)
Eculizumab is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy. Dosage:
- 900 mg weekly for the first 4 weeks, followed by
- 1200 mg for the fifth dose 1 week later, then
- 1200 mg every 2 weeks thereafter.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eculizumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eculizumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Atypical Hemolytic Uremic Syndrome (aHUS)
- Eculizumab is indicated for the treatment of patients with atypical hemolytic uremic syndrome (aHUS) to inhibit complement-mediated thrombotic microangiopathy.
- For patients less than 18 years of age, administer eculizumab based upon body weight, according to the following schedule:
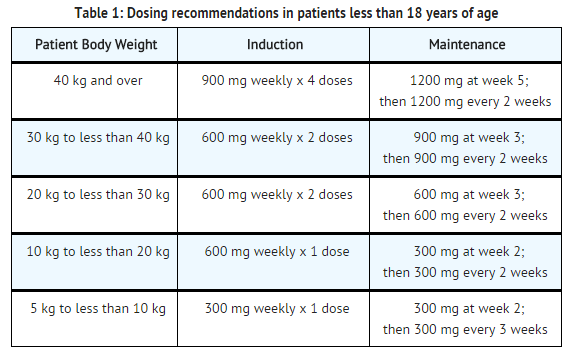
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eculizumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eculizumab in pediatric patients.
Contraindications
- Patients with unresolved serious Neisseria meningitidis infection.
- Patients who are not currently vaccinated against Neisseria meningitidis, unless the risks of delaying Soliris treatment outweigh the risks of developing a meningococcal infection
Warnings
|
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
See full prescribing information for complete Boxed Warning.
Life-threatening and fatal meningococcal infections have occurred in patients treated with eculizumab. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early.
|
Serious Meningococcal Infections
- The use of Soliris increases a patient's susceptibility to serious meningococcal infections (septicemia and/or meningitis). Life-threatening and fatal meningococcal infections have occurred in patients treated with Soliris.
- Administer a polyvalent meningococcal vaccine according to the most current Advisory Committee on Immunization Practices (ACIP) recommendations for patients with complement deficiencies. Revaccinate patients in accordance with ACIP recommendations, considering the duration of Soliris therapy.
- Immunize patients without a history of meningococcal vaccination at least 2 weeks prior to receiving the first dose of Soliris. If urgent Soliris therapy is indicated in an unvaccinated patient, administer the meningococcal vaccine as soon as possible. In prospective clinical studies, 75/100 patients with aHUS were treated with Soliris less than 2 weeks after meningococcal vaccination and 64 of these 75 patients received antibiotics for prophylaxis of meningococcal infection until at least 2 weeks after meningococcal vaccination. The benefits and risks of antibiotic prophylaxis for prevention of meningococcal infections in patients receiving Soliris have not been established.
- Vaccination reduces, but does not eliminate, the risk of meningococcal infections. In clinical studies, 2 out of 196 PNH patients developed serious meningococcal infections while receiving treatment with Soliris; both had been vaccinated. In clinical studies among non-PNH patients, meningococcal meningitis occurred in one unvaccinated patient. In addition, 3 out of 130 previously vaccinated patients with aHUS developed meningococcal infections while receiving treatment with Soliris.
- Closely monitor patients for early signs and symptoms of meningococcal infection and evaluate patients immediately if an infection is suspected. Meningococcal infection may become rapidly life-threatening or fatal if not recognized and treated early. Discontinue Soliris in patients who are undergoing treatment for serious meningococcal infections.
Soliris REMS
- Because of the risk of meningococcal infections, Soliris is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS). Under the Soliris REMS, prescribers must enroll in the program.
- Prescribers must counsel patients about the risk of meningococcal infection, provide the patients with the REMS educational materials, and ensure patients are vaccinated with a meningococcal vaccine.
Other Infections
- Soliris blocks terminal complement activation; therefore patients may have increased susceptibility to infections, especially with encapsulated bacteria. Additionally, Aspergillus infections have occurred in immunocompromised and neutropenic patients. Children treated with Soliris may be at increased risk of developing serious infections due to Streptococcuspneumoniae and Haemophilus influenza type b (Hib). Administer vaccinations for the prevention of Streptococcus pneumoniae and Haemophilus influenza type b (Hib) infections according to ACIP guidelines. Use caution when administering Soliris to patients with any systemic infection.
Monitoring Disease Manifestations After Soliris Discontinuation
Treatment Discontinuation for PNH
- Monitor patients after discontinuing Soliris for at least 8 weeks to detect hemolysis.
Treatment Discontinuation for aHUS
- After discontinuing Soliris, monitor patients with aHUS for signs and symptoms of thrombotic microangiopathy (TMA) complications for at least 12 weeks. In aHUS clinical trials, 18 patients (5 in the prospective studies) discontinued Soliris treatment. TMA complications occurred following a missed dose in 5 patients, and Soliris was reinitiated in 4 of these 5 patients.
Clinical signs and symptoms of TMA include changes in mental status, seizures, angina, dyspnea, or thrombosis. In addition, the following changes in laboratory parameters may identify a TMA complication: occurrence of two, or repeated measurement of any one of the following: a decrease in platelet count by 25% or more compared to baseline or the peak platelet count during Soliris treatment; an increase in serum creatinine by 25% or more compared to baseline or nadir during Soliris treatment; or, an increase in serum LDH by 25% or more over baseline or nadir during Soliris treatment.
If TMA complications occur after Soliris discontinuation, consider reinstitution of Soliris treatment, plasma therapy [plasmapheresis, plasma exchange, or fresh frozen plasma infusion (PE/PI)], or appropriate organ-specific supportive measures.
Thrombosis Prevention and Management
- The effect of withdrawal of anticoagulant therapy during Soliris treatment has not been established. Therefore, treatment with Soliris should not alter anticoagulant management.
Infusion Reactions
- As with all protein products, administration of Soliris may result in infusion reactions, including anaphylaxis or other hypersensitivity reactions. In clinical trials, no patients experienced an infusion reaction which required discontinuation of Soliris. Interrupt Soliris infusion and institute appropriate supportive measures if signs of cardiovascular instability or respiratory compromise occur.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Meningococcal infections are the most important adverse reactions experienced by patients receiving Soliris. In PNH clinical studies, two patients experienced meningococcal sepsis. Both patients had previously received a meningococcal vaccine. In clinical studies among patients without PNH, meningococcal meningitis occurred in one unvaccinated patient. Meningococcal sepsis occurred in one previously vaccinated patient enrolled in the retrospective aHUS study during the post-study follow-up period.
PNH
The data described below reflect exposure to Soliris in 196 adult patients with PNH, age 18-85, of whom 55% were female. All had signs or symptoms of intravascular hemolysis. Soliris was studied in a placebo-controlled clinical study (in which 43 patients received Soliris and 44, placebo); a single arm clinical study and a long term extension study. 182 patients were exposed for greater than one year. All patients received the recommended Soliris dose regimen.
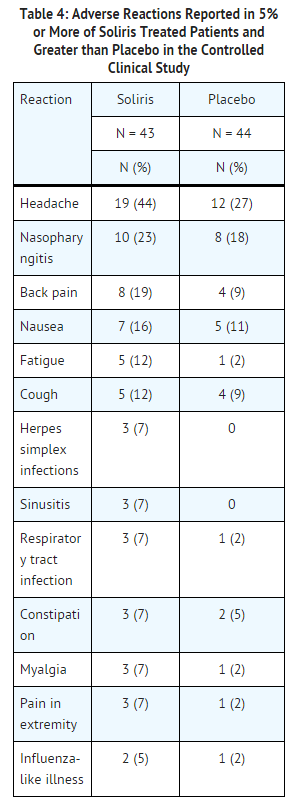
In the placebo-controlled clinical study, serious adverse reactions occurred among 4 (9%) patients receiving Soliris and 9 (21%) patients receiving placebo. The serious reactions included infections and progression of PNH. No deaths occurred in the study and no patients receiving Soliris experienced a thrombotic event; one thrombotic event occurred in a patient receiving placebo.
Among 193 patients with PNH treated with Soliris in the single arm, clinical study or the follow-up study, the adverse reactions were similar to those reported in the placebo-controlled clinical study. Serious adverse reactions occurred among 16% of the patients in these studies. The most common serious adverse reactions were: viral infection (2%), headache (2%), anemia (2%), and pyrexia (2%).
aHUS
The safety of Soliris therapy in patients with aHUS was evaluated in four prospective, single-arm studies, three in adult and adolescent patients (aHUS Studies 1, 2, and 4), one in pediatric and adolescent patients (aHUS Study 5) and one retrospective study (aHUS Study 3).
The data described below were derived from 78 adult and adolescent patients with aHUS enrolled in aHUS Study 1, aHUS Study 2, and aHUS Study 4. All patients received the recommended dosage of Soliris. Median exposure was 67 weeks (range: 2-145 weeks). Table 5 summarizes all adverse events reported in at least 10% of patients in aHUS Studies 1, 2, and 4 combined.

In aHUS Studies 1, 2, and 4 combined, 60% (47/78) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were infections (24%), hypertension (5%), chronic renal failure (5%), and renal impairment (5%). Five patients discontinued Soliris due to adverse events; three due to worsening renal function, one due to new diagnosis of Systemic Lupus Erythematosus, and one due to meningoccal meningitis.
aHUS Study 5 included 22 pediatric and adolescent patients, of which 18 patients were less than 12 years of age. All patients received the recommended dosage of Soliris. Median exposure was 44 weeks (range: 1 dose-87 weeks).
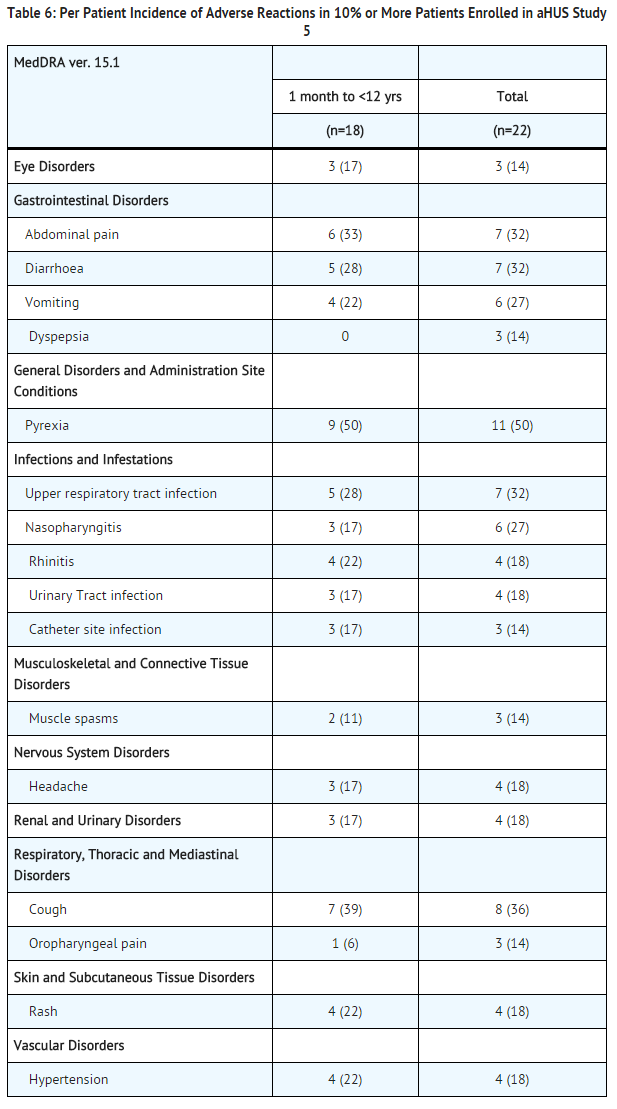
In aHUS Study 5, 59% (13/22) of patients experienced a serious adverse event (SAE). The most commonly reported SAEs were hypertension (9%), viral gastroenteritis (9%), pyrexia (9%), and upper respiratory infection (9%). One patient discontinued Soliris due to an adverse event (severe agitation).
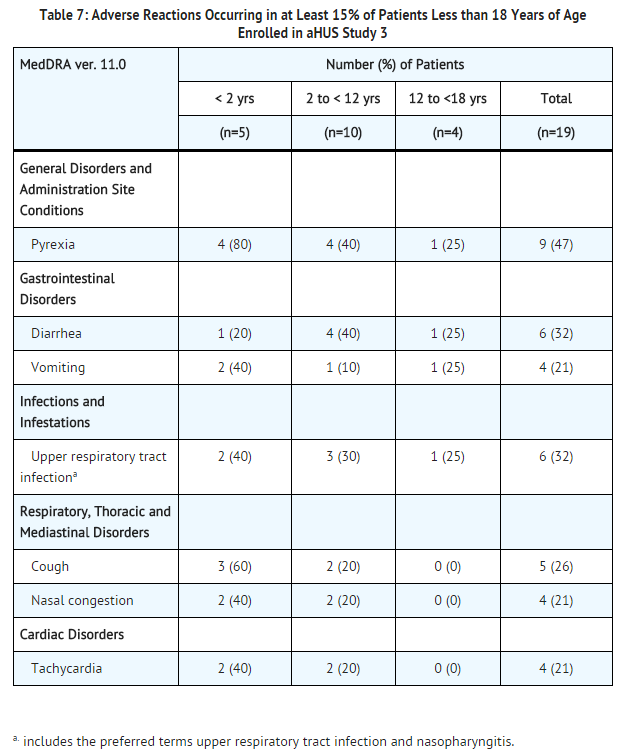
Analysis of retrospectively collected adverse event data from pediatric and adult patients enrolled in aHUS Study 3 (N=30) revealed a safety profile that was similar to that which was observed in the two prospective studies. aHUS Study 3 included 19 pediatric patients less than 18 years of age. Overall, the safety of Soliris in pediatric patients with aHUS enrolled in Study 3 appeared similar to that observed in adult patients. The most common (≥15%) adverse events occurring in pediatric patients are presented in Table 7.
Immunogenicity
As with all proteins, there is a potential for immunogenicity with eculizumab. The immunogenicity of Soliris has been evaluated using two different immunoassays for the detection of anti-eculizumab antibodies: a direct enzyme-linked immunosorbent assay (ELISA) using the Fab fragment of eculizumab as target was used for the PNH indication; and an electro-chemiluminescence (ECL) bridging assay using the eculizumab whole molecule as target was used for the aHUS indication, as well as for additional patients with PNH. In the PNH population, antibodies to Soliris were detected in 3/196 (2%) patients with PNH treated with Soliris using the ELISA assay and in 5/161 (3%) patients treated with Soliris using the ECL assay. In patients with aHUS treated with Soliris, antibodies to Soliris were detected in 3/100 (3%) using the ECL assay. An ECL based neutralizing HAHA assay with a low sensitivity of 2 mcg/mL was performed to detect neutralizing antibodies for the 3 patients with aHUS and also for the 5 patients with PNH with positive samples using the ECL assay. 2/161 patients in the PNH group (1.2%) and 1/100 patients in the aHUS group (1%) had low positive values for neutralizing antibodies. No apparent correlation of antibody development to clinical response was observed in either indication. The immunogenicity data reflect the percentage of patients whose test results were considered positive for antibodies to Soliris in an ELISA-based assay and/or an ECL-based assay and are highly dependent on the sensitivity and specificity of the assay used. Additionally, the observed incidence of antibody positivity in the assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to Soliris with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Soliris. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Soliris exposure.
Cases of serious or fatal meningococcal infections have been reported.
Drug Interactions
Drug interaction studies have not been performed with Soliris.
Use in Specific Populations
Pregnancy
Risk Summary
There are no adequate and well-controlled studies of Soliris in pregnant women. Soliris, a recombinant IgG molecule (humanized anti-C5 antibody), is expected to cross the placenta. Animal studies using a mouse analogue of the Soliris molecule (murine anti-C5 antibody) showed increased rates of developmental abnormalities and an increased rate of dead and moribund offspring at doses 2-8 times the human dose. Soliris should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
Animal reproduction studies were conducted in mice using doses of a murine anti-C5 antibody that approximated 2-4 times (low dose) and 4-8 times (high dose) the recommended human Soliris dose, based on a body weight comparison. When animal exposure to the antibody occurred in the time period from before mating until early gestation, no decrease in fertility or reproductive performance was observed. When maternal exposure to the antibody occurred during organogenesis, two cases of retinal dysplasia and one case of umbilical hernia were observed among 230 offspring born to mothers exposed to the higher antibody dose; however, the exposure did not increase fetal loss or neonatal death. When maternal exposure to the antibody occurred in the time period from implantation through weaning, a higher number of male offspring became moribund or died (1/25 controls, 2/25 low dose group, 5/25 high dose group). Surviving offspring had normal development and reproductive function.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Eculizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Eculizumab during labor and delivery.
Nursing Mothers
It is not known whether Soliris is excreted into human milk. IgG is excreted in human milk, so it is expected that Soliris will be present in human milk. However, published data suggest that antibodies in human milk do not enter the neonatal and infant circulation in substantial amounts. Caution should be exercised when Soliris is administered to a nursing woman. The unknown risks to the infant from gastrointestinal or limited systemic exposure to Soliris should be weighed against the known benefits of human milk feeding.
Pediatric Use
The safety and effectiveness of Soliris for the treatment of PNH in pediatric patients below the age of 18 years have not been established.
Four clinical studies assessing the safety and effectiveness of Soliris for the treatment of aHUS included a total of 47 pediatric patients (ages 2 months to 17 years). The safety and effectiveness of Soliris for the treatment of aHUS appear similar in pediatric and adult patients.
Administer vaccinations for the prevention of infection due to Neisseria meningitidis, Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to ACIP guidelines.
Geriatic Use
Nineteen patients 65 years of age or older (15 with PNH and 4 with aHUS) were treated with Soliris. Although there were no apparent age-related differences observed in these studies, the number of patients aged 65 and over is not sufficient to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Eculizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Eculizumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Eculizumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Eculizumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
Effects of eculizumab upon fertility have not been studied in animals. Intravenous injections of male and female mice with a murine anti-C5 antibody at up to 4-8 times the equivalent of the clinical dose of Soliris had no adverse effects on mating or fertility.
Immunocompromised Patients
There is no FDA guidance one the use of Eculizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Eculizumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Eculizumab and IV administrations.
Overdosage
No cases of Soliris overdose have been reported during clinical studies.
Pharmacology
Eculizumab?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | Complement protein C5 |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | 148 kDa |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 8 to 15 days (mean 11 days) |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
C(US) |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous infusion |
Mechanism of Action
Eculizumab, the active ingredient in Soliris, is a monoclonal antibody that specifically binds to the complement protein C5 with high affinity, thereby inhibiting its cleavage to C5a and C5b and preventing the generation of the terminal complement complex C5b-9. Soliris inhibits terminal complement mediated intravascular hemolysis in PNH patients and complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS.
A genetic mutation in patients with PNH leads to the generation of populations of abnormal RBCs (known as PNH cells) that are deficient in terminal complement inhibitors, rendering PNH RBCs sensitive to persistent terminal complement-mediated destruction. The destruction and loss of these PNH cells (intravascular hemolysis) results in low RBC counts (anemia), and also fatigue, difficulty in functioning, pain, dark urine, shortness of breath, and blood clots.
In aHUS, impairment in the regulation of complement activity leads to uncontrolled terminal complement activation, resulting in platelet activation, endothelial cell damage and thrombotic microangiopathy.
Structure
Eculizumab is composed of two 448 amino acid heavy chains and two 214 amino acid light chains and has a molecular weight of approximately 148 kDa.
Pharmacodynamics
In the PNH placebo-controlled clinical study, Soliris when administered as recommended reduced hemolysis as shown by the reduction of serum LDH levels from 2200 ± 1034 U/L (mean ± SD) at baseline to 700 ± 388 U/L by week one and maintained the effect through the end of the study at week 26 (327 ± 433 U/L). In the single arm clinical study, Soliris maintained this effect through 52 weeks.
Pharmacokinetics
A population PK analysis with a standard 1-compartmental model was conducted on the multiple dose PK data from 40 PNH patients receiving the recommended Soliris regimen. In this model, the clearance of Soliris for a typical PNH patient weighing 70 kg was 22 mL/hr and the volume of distribution was 7.7 L. The half-life was 272 ± 82 hrs (mean ± SD). The mean observed peak and trough serum concentrations of Soliris by week 26 were 194 ± 76 mcg/mL and 97 ± 60 mcg/mL, respectively.
A second population PK analysis with a standard 1 compartmental model was conducted on the multiple dose PK data from 57 aHUS patients receiving the recommended Soliris regimen in studies 1, 2 and 3. In this model, the clearance of Soliris for a typical aHUS patient weighing 70 kg was 14.6 mL/hr and the volume of distribution was 6.14 L. The elimination half-life was 291 h (approximately 12.1 days).
The clearance and half-life of eculizumab were also evaluated during plasma exchange interventions. Plasma exchange increased the clearance of eculizumab to 3660 mL/hr and reduced the half-life to 1.26 hours. Supplemental dosing is recommended when Soliris is administered to aHUS patients receiving plasma infusion or exchange.
Dedicated studies have not been conducted to evaluate the PK of Soliris in special patient populations identified by gender, race, age (geriatric), or the presence of renal or hepatic impairment. Pediatric and adolescent patients (less than 18 years of age) and patients with renal impairment were included in the aHUS clinical studies. Population PK analysis showed age, gender, race, and renal function do not influence the PK of eculizumab.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis
- Long-term animal carcinogenicity studies of eculizumab have not been conducted.
- Genotoxicity studies have not been conducted with eculizumab.
Clinical Studies
PNH
The safety and efficacy of Soliris in PNH patients with hemolysis were assessed in a randomized, double-blind, placebo-controlled 26 week study (Study 1); PNH patients were also treated with Soliris in a single arm 52 week study (Study 2); and in a long term extension study. Patients received meningococcal vaccination prior to receipt of Soliris. In all studies, the dose of Soliris was 600 mg study drug every 7 ± 2 days for 4 weeks, followed by 900 mg 7 ± 2 days later, then 900 mg every 14 ± 2 days for the study duration. Soliris was administered as an intravenous infusion over 25 - 45 minutes.
Study 1
PNH patients with at least four transfusions in the prior 12 months, flow cytometric confirmation of at least 10% PNH cells and platelet counts of at least 100,000/microliter were randomized to either Soliris (n = 43) or placebo (n = 44). Prior to randomization, all patients underwent an initial observation period to confirm the need for RBC transfusion and to identify the hemoglobin concentration (the "set-point") which would define each patient's hemoglobin stabilization and transfusion outcomes. The hemoglobin set-point was less than or equal to 9 g/dL in patients with symptoms and was less than or equal to 7 g/dL in patients without symptoms. Endpoints related to hemolysis included the numbers of patients achieving hemoglobin stabilization, the number of RBC units transfused, fatigue, and health-related quality of life. To achieve a designation of hemoglobin stabilization, a patient had to maintain a hemoglobin concentration above the hemoglobin set-point and avoid any RBC transfusion for the entire 26 week period. Hemolysis was monitored mainly by the measurement of serum LDH levels, and the proportion of PNH RBCs was monitored by flow cytometry. Patients receiving anticoagulants and systemic corticosteroids at baseline continued these medications.
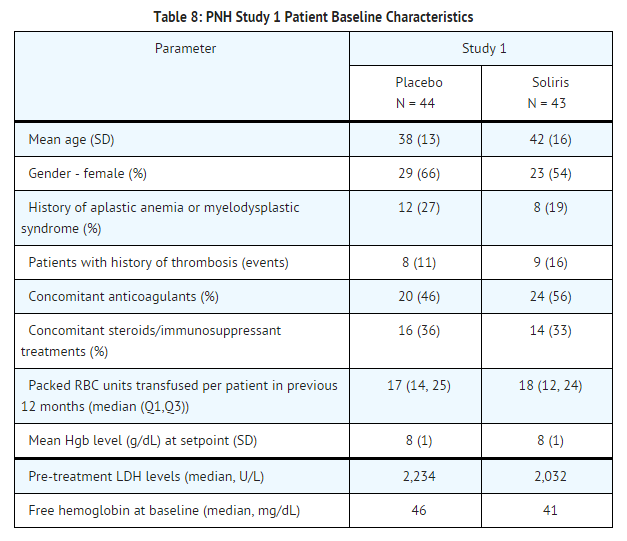
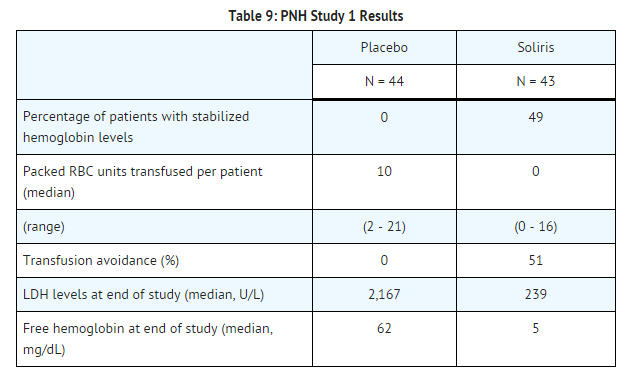
Patients treated with Soliris had significantly reduced (p< 0.001) hemolysis resulting in improvements in anemia as indicated by increased hemoglobin stabilization and reduced need for RBC transfusions compared to placebo treated patients (see Table 9). These effects were seen among patients within each of the three pre-study RBC transfusion strata (4 - 14 units; 15 - 25 units; > 25 units). After 3 weeks of Soliris treatment, patients reported less fatigue and improved health-related quality of life. Because of the study sample size and duration, the effects of Soliris on thrombotic events could not be determined.
Percentage of patients with stabilized hemoglobin levels:
Study 2 and Extension Study
PNH patients with at least one transfusion in the prior 24 months and at least 30,000 platelets/microliter received Soliris over a 52-week period. Concomitant medications included anti-thrombotic agents in 63% of the patients and systemic corticosteroids in 40% of the patients. Overall, 96 of the 97 enrolled patients completed the study (one patient died following a thrombotic event). A reduction in intravascular hemolysis as measured by serum LDH levels was sustained for the treatment period and resulted in a reduced need for RBC transfusion and less fatigue. 187 Soliris-treated PNH patients were enrolled in a long term extension study. All patients sustained a reduction in intravascular hemolysis over a total Soliris exposure time ranging from 10 to 54 months. There were fewer thrombotic events with Soliris treatment than during the same period of time prior to treatment. However, the majority of patients received concomitant anticoagulants; the effects of anticoagulant withdrawal during Soliris therapy was not studied.
aHUS
Five single-arm studies [four prospective (aHUS Studies 1, 2, 4 and 5) and one retrospective (aHUS Study 3)] evaluated the safety and efficacy of Soliris for the treatment of aHUS. Patients with aHUS received meningococcal vaccination prior to receipt of Soliris or received prophylactic treatment with antibiotics until 2 weeks after vaccination. In all studies, the dose of Soliris in adult and adolescent patients was 900 mg every 7 ± 2 days for 4 weeks, followed by 1200 mg 7 ± 2 days later, then 1200 mg every 14 ± 2 days thereafter. The dosage regimen for pediatric patients weighing less than 40 kg enrolled in aHUS Study 3 and Study 5 was based on body weight. Efficacy evaluations were based on thrombotic microangiopathy (TMA) endpoints.
Endpoints related to TMA included the following:
- platelet count change from baseline
- hematologic normalization (maintenance of normal platelet counts and LDH levels for at least four weeks)
- complete TMA response (hematologic normalization plus at least a 25% reduction in serum creatinine for a minimum of four weeks)
- TMA-event free status (absence for at least 12 weeks of a decrease in platelet count of >25% from baseline, plasma exchange or plasma infusion, and new dialysis requirement)
- Daily TMA intervention rate (defined as the number of plasma exchange or plasma infusion interventions and the number of new dialyses required per patient per day).
aHUS Resistant to PE/PI (aHUS Study 1)
aHUS Study 1 enrolled patients who displayed signs of thrombotic microangiopathy (TMA) despite receiving at least four PE/PI treatments the week prior to screening. One patient had no PE/PI the week prior to screening because of PE/PI intolerance. In order to qualify for enrollment, patients were required to have a platelet count ≤150 x 109/L, evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 28 (range: 17 to 68 years). Patients enrolled in aHUS Study 1 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 70%-121%. Seventy-six percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 10 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 1.
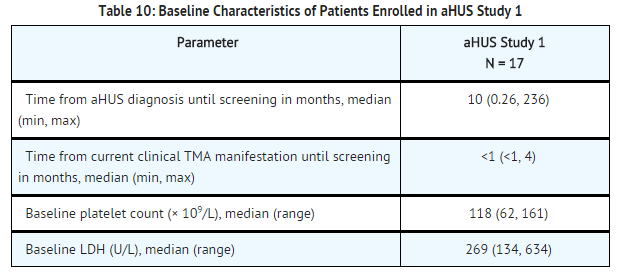
Patients in aHUS Study 1 received Soliris for a minimum of 26 weeks. In aHUS Study 1, the median duration of Soliris therapy was approximately 100 weeks (range: 2 weeks to 145 weeks).
Renal function, as measured by eGFR, was improved and maintained during Soliris therapy. The mean eGFR (± SD) increased from 23 ± 15 mL/min/1.73m2 at baseline to 56 ± 40 mL/min/1.73m2 by 26 weeks; this effect was maintained through 2 years (56 ± 30 mL/min/1.73m2). Four of the five patients who required dialysis at baseline were able to discontinue dialysis.
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 1, mean platelet count (± SD) increased from 109 ± 32 x109/L at baseline to 169 ± 72 x109/L by one week; this effect was maintained through 26 weeks (210 ± 68 x109/L), and 2 years (205 ± 46 x109/L). When treatment was continued for more than 26 weeks, two additional patients achieved Hematologic Normalization as well as Complete TMA response. Hematologic Normalization and Complete TMA response were maintained by all responders. In aHUS Study 1, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins.

aHUS Sensitive to PE/PI (aHUS Study 2)
aHUS Study 2 enrolled patients undergoing chronic PE/PI who generally did not display hematologic signs of ongoing thrombotic microangiopathy (TMA). All patients had received PT at least once every two weeks, but no more than three times per week, for a minimum of eight weeks prior to the first Soliris dose. Patients on chronic dialysis were permitted to enroll in aHUS Study 2. The median patient age was 28 years (range: 13 to 63 years). Patients enrolled in aHUS Study 2 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 37%-118%. Seventy percent of patients had an identified complement regulatory factor mutation or auto-antibody. Table 12 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 2.
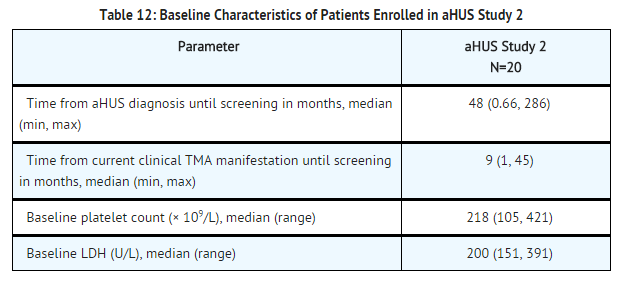
Patients in aHUS Study 2 received Soliris for a minimum of 26 weeks. In aHUS Study 2, the median duration of Soliris therapy was approximately 114 weeks (range: 26 to 129 weeks).
Renal function, as measured by eGFR, was maintained during Soliris therapy. The mean eGFR (± SD) was 31 ± 19 mL/min/1.73m2 at baseline, and was maintained through 26 weeks (37 ± 21 mL/min/1.73m2) and 2 years (40 ± 18 mL/min/1.73m2). No patient required new dialysis with Soliris.
Reduction in terminal complement activity was observed in all patients after the commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. Platelet counts were maintained at normal levels despite the elimination of PE/PI. The mean platelet count (± SD) was 228 ± 78 x 109/L at baseline, 233 ± 69 x 109/L at week 26, and 224 ± 52 x 109/L at 2 years. When treatment was continued for more than 26 weeks, six additional patients achieved Complete TMA response. Complete TMA Response and Hematologic Normalization were maintained by all responders. In aHUS Study 2, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins.
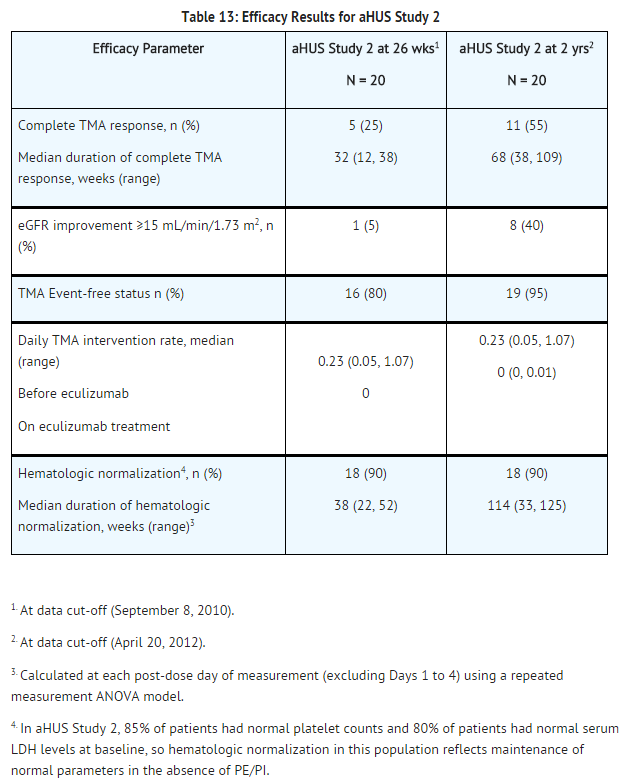
Retrospective Study in Patients with aHUS (aHUS Study 3)
The efficacy results for the aHUS retrospective study (aHUS Study 3) were generally consistent with results of the two prospective studies. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline. Mean platelet count (± SD) increased from 171 ± 83 x109/L at baseline to 233 ±109 x109/L after one week of therapy; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 254 ± 79 x109/L).
A total of 19 pediatric patients (ages 2 months to 17 years) received Soliris in aHUS Study 3. The median duration of Soliris therapy was 16 weeks (range 4 to 70 weeks) for children <2 years of age (n=5), 31 weeks (range 19 to 63 weeks) for children 2 to <12 years of age (n=10), and 38 weeks (range 1 to 69 weeks) for patients 12 to <18 years of age (n=4). Fifty three percent of pediatric patients had an identified complement regulatory factor mutation or auto-antibody.
Overall, the efficacy results for these pediatric patients appeared consistent with what was observed in patients enrolled in aHUS Studies 1 and 2 (Table 14). No pediatric patient required new dialysis during treatment with Soliris.
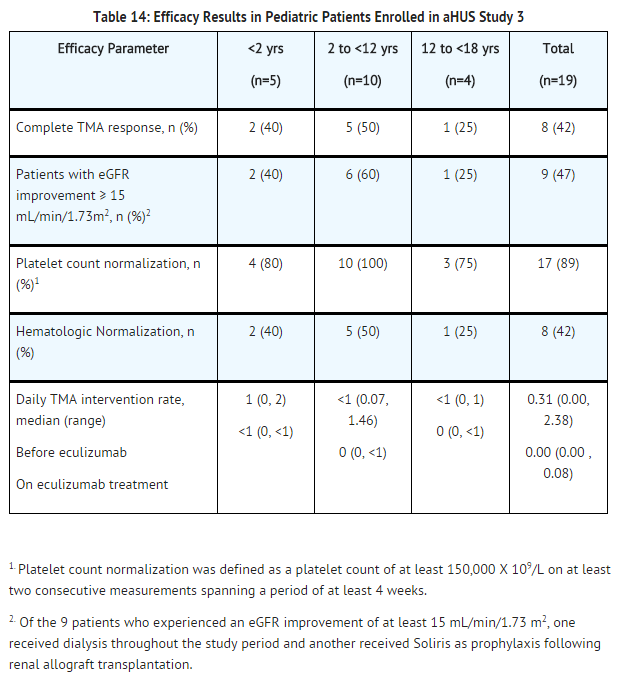
Adult Patients with aHUS (aHUS Study 4)
aHUS Study 4 enrolled patients who displayed signs of thrombotic microangiopathy (TMA). In order to qualify for enrollment, patients were required to have a platelet count < lower limit of normal range (LLN), evidence of hemolysis such as an elevation in serum LDH, and serum creatinine above the upper limits of normal, without the need for chronic dialysis. The median patient age was 35 (range: 18 to 80 years). All patients enrolled in aHUS Study 4 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 28%-116%. Fifty-one percent of patients had an identified complement regulatory factor mutation or auto-antibody. A total of 35 patients received PE/PI prior to eculizumab. Table 15 summarizes the key baseline clinical and disease-related characteristics of patients enrolled in aHUS Study 4.

Patients in aHUS Study 4 received Soliris for a minimum of 26 weeks. In aHUS Study 4, the median duration of Soliris therapy was approximately 50 weeks (range: 13 weeks to 86 weeks).
Renal function, as measured by eGFR, was improved during Soliris therapy. The mean eGFR (± SD) increased from 17 ± 12 mL/min/1.73m2 at baseline to 47 ± 24 mL/min/1.73m2 by 26 weeks. Twenty of the 24 patients who required dialysis at study baseline were able to discontinue dialysis during Soliris treatment.
Reduction in terminal complement activity and an increase in platelet count relative to baseline were observed after commencement of Soliris. Soliris reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. In aHUS Study 4, mean platelet count (± SD) increased from 119 ± 66 x109/L at baseline to 200 ± 84 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (± SD) at week 26: 252 ± 70 x109/L). In aHUS Study 4, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H.
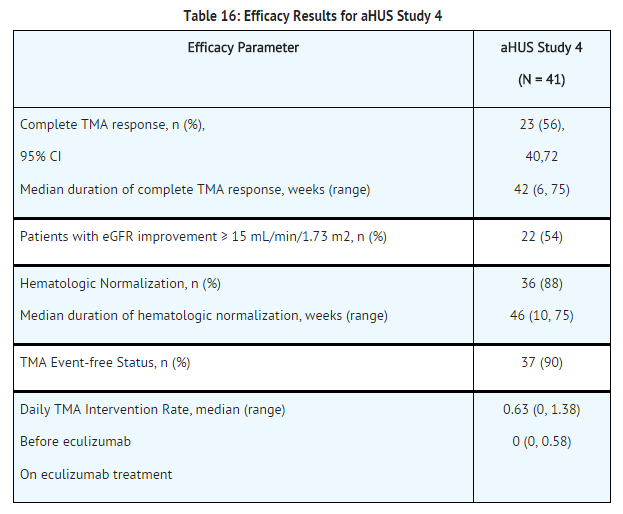
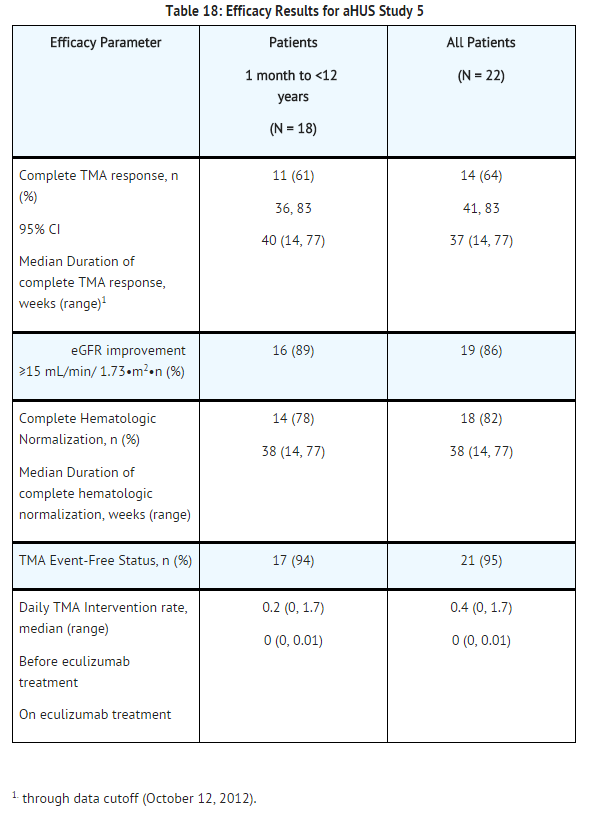
Pediatric and Adolescent Patients with aHUS (aHUS Study 5)
aHUS Study 5 enrolled patients who were required to have a platelet count < lower limit of normal range (LLN), evidence of hemolysis such as an elevation in serum LDH above the upper limits of normal, serum creatinine level ≥97 percentile for age without the need for chronic dialysis. The median patient age was 6.5 (range: 5 months to 17 years). Patients enrolled in aHUS Study 5 were required to have ADAMTS13 activity level above 5%; observed range of values in the trial were 38%-121%. Fifty percent of patients had an identified complement regulatory factor mutation or auto-antibody. A total of 10 patients received PE/PI prior to eculizumab.
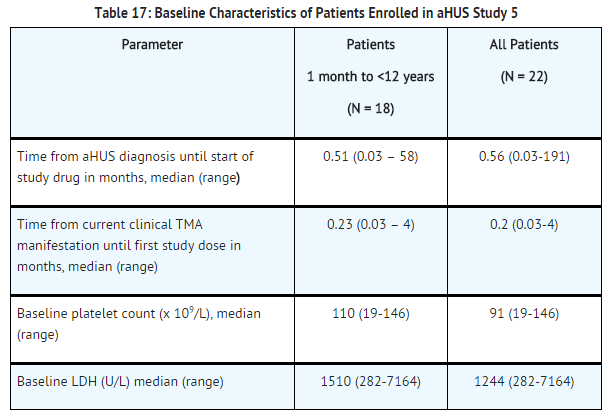
Patients in aHUS Study 5 received Soliris for a minimum of 26 weeks. In aHUS Study 5, the median duration of Soliris therapy was approximately 44 weeks (range: 1 dose to 88 weeks).
Renal function, as measured by eGFR, was improved during Soliris therapy. The mean eGFR (± SD) increased from 33 ± 30 mL/min/1.73m2 at baseline to 98 ± 44 mL/min/1.73m2 by 26 weeks. Among the 20 patients with a CKD stage ≥2 at baseline, 17 (85%) achieved a CKD improvement of ≥1 stage. Among the 16 patients ages 1 month to <12 years with a CKD stage ≥2 at baseline, 14 (88%) achieved a CKD improvement by ≥1 stage. Nine of the 11 patients who required dialysis at study baseline were able to discontinue dialysis during Soliris treatment. Responses were observed across all ages from 5 months to 17 years of age.
Reduction in terminal complement activity was observed in all patients after commencement of Soliris. Eculizumab reduced signs of complement-mediated TMA activity, as shown by an increase in mean platelet counts from baseline to 26 weeks. The mean platelet count (± SD) increased from 88 ± 42 x109/L at baseline to 281 ± 123 x109/L by one week; this effect was maintained through 26 weeks (mean platelet count (±SD) at week 26: 293 ± 106 x109/L). In aHUS Study 5, responses to Soliris were similar in patients with and without identified mutations in genes encoding complement regulatory factor proteins or auto-antibodies to factor H.
How Supplied
Eculizumab is supplied as 300 mg single-use vials containing 30 mL of 10 mg/mL sterile, preservative-free Soliris solution per vial.
Storage
Store at 2-8º C (36-46º F) and protected from light.
Images
Drug Images
{{#ask: Page Name::Eculizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eculizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Prior to treatment, patients should fully understand the risks and benefits of Soliris, in particular the risk of meningococcal infection. Ensure that patients receive the Medication Guide.
Inform patients that they are required to receive a meningococcal vaccination at least 2 weeks prior to receiving the first dose of Soliris, if they have not previously been vaccinated. They are required to be revaccinated according to current medical guidelines for meningococcal vaccine use while on Soliris therapy. Inform patients that vaccination may not prevent meningococcal infection. Inform patients about the signs and symptoms of meningococcal infection, and strongly advise patients to seek immediate medical attention if these signs or symptoms occur. These signs and symptoms are as follows:
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- fever of 103° F (39.4° C) or higher
- fever and a rash
- confusion
- muscle aches with flu-like symptoms
- eyes sensitive to light
Inform patients that they will be given a Soliris Patient Safety Information Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the patient to immediately seek medical evaluation.
Inform patients that there may be an increased risk of other types of infections, particularly those due to encapsulated bacteria. Additionally, Aspergillus infections have occured in immunocompromised and neutropenic patients. Inform parents or caregivers of children receiving Soliris for the treatment of aHUS that their child should be vaccinated against Streptococcus pneumoniae and Haemophilus influenza type b (Hib) according to current medical guidelines.
Inform patients with PNH that they may develop hemolysis due to PNH when Soliris is discontinued and that they will be monitored by their healthcare professional for at least 8 weeks following Soliris discontinuation. Inform patients with aHUS that there is a potential for TMA complications due to aHUS when Soliris is discontinued and that they will be monitored by their healthcare professional for at least 12 weeks following Soliris discontinuation. Inform patients who discontinue Soliris to keep the Soliris Patient Safety Information Card with them for three months after the last Soliris dose, because the increased risk of meningococcal infection persists for several weeks following discontinuation of Soliris.
Precautions with Alcohol
Alcohol-Eculizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Eculizumab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Eculizumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Template:Infobox drug/mab source |
| Target | Complement protein C5 |
| Clinical data | |
| Trade names | Soliris |
| AHFS/Drugs.com | Monograph |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 8 to 15 days (mean 11 days) |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Molar mass | 148 kDa |
| | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Eculizumab (INN and USAN; trade name Soliris) is a humanized monoclonal antibody that is a first-in-class terminal complement inhibitor and the first therapy approved for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare, progressive, and sometimes life-threatening disease characterized by excessive destruction of red blood cells (hemolysis).[1] It costs approximately £245,700 for ongoing treatment, but there is variance depending on clinical response.[2]
Eculizumab also is the first agent approved for the treatment of atypical hemolytic uremic syndrome (aHUS), an ultra-rare genetic disease that causes abnormal blood clots to form in small blood vessels throughout the body, leading to kidney failure, damage to other vital organs and premature death.[3][4]
In clinical trials in patients with PNH, eculizumab was associated with reductions in chronic hemolysis, thromboembolic events, and transfusion requirements, as well as improvements in PNH symptoms, quality of life, and survival.[1][5][6][7] Clinical trials in patients with aHUS demonstrated inhibition of thrombotic microangiopathy (TMA),[8] the formation of blood clots in small blood vessels throughout the body,[1][4][5] including normalization of platelets and lactate dehydrogenase (LDH), as well as maintenance or improvement in renal function.[8]
Eculizumab was discovered and developed by Alexion Pharmaceuticals and is manufactured by Alexion. It was approved by the United States Food and Drug Administration (FDA) on March 16, 2007 for the treatment of PNH, and on September 23, 2011 for the treatment of aHUS. It was approved by the European Medicines Agency for the treatment of PNH on June 20, 2007, and on November 24, 2011 for the treatment of aHUS. Eculizumab is currently being investigated as a potential treatment for other severe, ultra-rare disorders.
Medical Uses
Paroxysmal Nocturnal Hemoglobinuria
In those with paroxysmal nocturnal hemoglobinuria despite best historical care (e.g., anticoagulation therapy, transfusions), 35% of patients with PNH die within 5 years of diagnosis.[9] Thromboembolism (TE) is the leading cause of death in PNH patients, accounting for 40% to 67% of PNH-related mortality.[10][11] Patients with PNH are 62 times more likely to have a venous thromboembolism than the general population, which is higher than any other hyper-coagulable disease.[12][13] Both venous and arterial thromboembolism can occur in patients with PNH.[14] Although deep vein thrombosis and pulmonary embolism are the most common clinical presentations,[10] thrombosis in atypical sites (including Budd-Chiari, renal, and dermal thrombosis) is more common among PNH patients than in the general population. In a national registry, 39% of TEs in patients with PNH occurred in arterial sites.[15] For patients who experience TE and survive, the risk of subsequent TEs is increased,[10] and the risk of death increases 5- to 15-fold for PNH patients with no previous TE.[15] Notably, data suggest 60% of patients with PNH have evidence of undiagnosed thrombosis.[12] In one analysis, thromboembolism was observed in a significantly higher rate of PNH patients with LDH levels over 1.5 times the upper limit of normal.[16]
Eculizumab protects blood cells against immune destruction by inhibiting the complement system. In the TRIUMPH study, a double-blind, randomized, placebo-controlled, Phase III trial involving 87 patients, eculizumab was associated with an 86% reduction in intravascular hemolysis, as measured by LDH reduction, as well as a 73% reduction in transfusions across all subgroups. Notably, there was a rapid and meaningful improvement in fatigue and quality of life for the treated patients. No serious treatment-related adverse events were reported.[1]
Significantly, data from three independent clinical studies—a Phase II pilot study,[5] the Phase III TRIUMPH study,[1] and the Phase III SHEPHERD study[6]—demonstrated a greater than 90% reduction in thromboembolic events, the most serious complication of PNH and a major cause of death in this disease. All PNH patients in clinical trials derived a benefit from eculizumab therapy; many benefits occurred rapidly, while others showed improvement over time.[8]
aHUS
Historically, 33-40% of patients with aHUS died or progressed to ESRD with the first clinical manifestation of the disease.[3][4] Moreover, 65% of all patients with aHUS will die, require kidney dialysis, or have permanent renal damage within one year of diagnosis despite administration of plasma exchange or plasma infusion (PE/PI).[4] Prior to the availability of eculizumab, management of aHUS did not specifically target chronic uncontrolled complement activation, the underlying cause of systemic TMA in aHUS. Not only is PE/PI ineffective in arresting platelet activation and systemic TMA, but it also causes poor outcomes and carries the risk of serious – and sometimes fatal – complications.[17][18] Even those patients who survive the first clinical manifestations of aHUS continue to experience TMA and remain at risk of progressive failure of vital organs or sudden death.[4]
Eculizumab has been shown to inhibit terminal complement activity in children and adults with aHUS, and to eliminate the need for PE/PI and new dialysis.[8] The efficacy and safety of eculizumab in aHUS have been studied in two prospective studies, one involving 17 patients with progressing TMA (median age 28 years, range 17-68) who were resistant to or intolerant of PE/PI (Study 1)[21], and the other involving 20 patients with long duration of aHUS (median age 28 years, range 13–63; median duration from diagnosis of aHUS to screening 48 months) who were receiving chronic PE/PI (Study 2).[19] TMA-related endpoints in these trials included the following:[8][19][20]
- Platelet count change from baseline
- Hematologic normalization (maintenance of normal platelet counts and lactate dehydrogenase [LDH] levels for at least four weeks)
- Complete TMA response (hematologic normalization plus at least a 25% reduction in serum creatininefor a minimum of four weeks)
- TMA-event free status (absence for at least 12 weeks of a decrease in platelet count of >25% from baseline, PE/PI, and new dialysis requirement)
- Daily TMA intervention rate (defined as the number of PE/PI interventions and the number of new dialyses required per patient per day)
- Time course of changes in renal function as measured by eGFR
- Proportion of patients with improvement by ≥ 1 chronic kidney disease (CKD) stage, eGFR increase by ≥ 15 mL/min/1.73m2 or serum creatinine decrease by ≥25%
In Study 1, eculizumab inhibited complement-mediated TMA activity in all 17 patients through 26 weeks. Efficacy findings included a significant and sustained increase in platelet count through Week 26. Thirteen patients (76%) achieved hematologic normalization, and 15 patients (88%) achieved TMA event-free status. Renal function, as measured by eGFR, improved in nine patients (53%); the median duration of eGFR improvement was 251 days. Additionally, four of the five patients requiring dialysis at study entry were able to discontinue dialysis for the duration of eculizumab treatment. Quality of life (QoL) was significantly improved, with 80% of patients achieving a clinically meaningful change through Week 26; this increased to 87% through 1 year.[8][20]
Similar results were reported in Study 2, in which 16 patients (80%) achieved TMA event-free status and 18 (90%) achieved hematologic normalization. All patients discontinued PE/PI and no new dialysis was required. Eculizumab was associated with mean increases in platelet count and eGFR from baseline to 26 weeks. QoL was also improved, with 8 of 11 (73%) evaluable patients exceeding the clinically meaningful threshold through a median duration of 62 weeks.[8][19]
After completing the initial 26-week treatment period, most patients in each of the prospective studies continued to receive eculizumab by enrolling in an extension study. Two-year follow-up data from the extension studies suggest that eculizumab provides sustained inhibition of complement-mediated TMA and significant, continuous, time-dependent improvement in renal function. In the extension to Study 1, in which 13 patients were treated for a median duration of 100 weeks, chronic eculizumab treatment resulted in a continued increase in platelet counts and greater percentages of patients achieving key renal endpoints compared to those completing 26 weeks of therapy. Additionally, at a median duration of almost 2 years, all patients receiving chronic eculizumab therapy remain alive.[21] In the extension to Study 2, in which 20 patients were treated for a median duration of 114 weeks, 19 (95%) achieved TMA event-free status, and the percentages of patients reaching the key renal endpoints were also higher than at Week 26. No patient on chronic eculizumab therapy in the extension to Study 2 required PE/PI or progressed to ESRD or dialysis.[22]
Efficacy results from a single-arm retrospective study, in which 30 patients (including 19 pediatric patients aged 2 months to 17 years) were treated for a median of 16 weeks, were generally consistent with those from the two prospective studies. Among the pediatric patients, eculizumab reduced signs of TMA activity, as shown by an increase in mean platelet counts from baseline. Seventeen (89%) pediatric patients achieved platelet count normalization, 8 (42%) attained hematologic normalization, 8 (42%) had a complete TMA response, and 9 (47%) experienced improvement in eGFR from baseline. Four of 8 pediatric patients (50%) discontinued dialysis during the study period, and none required new dialysis while on eculizumab therapy.[8]
In addition to the above single-arm studies, there are dozens of published case reports of the use of eculizumab in patients with aHUS, including several reporting complete or partial recovery of renal function with no need for subsequent kidney replacement therapy.[23] One pediatric patient, who was initially diagnosed shortly after birth and experienced four episodes of clinical TMA complications within 18 months following discontinuation of PE/PI, has been treated with eculizumab for 36 months as of December 2011, with no evidence of aHUS clinical manifestation, adverse events, or serious infections; this is the longest reported event-free period in the clinical literature.[24]
Adverse Effects
In PNH clinical trials, the most frequently reported adverse events (AEs) were headache (44%), nasopharyngitis (23%), back pain (19%), nausea (16%), fatigue (12%), and cough (12%).[8] In two prospective clinical trials in aHUS, the most commonly reported AEs were hypertension (35%), upper respiratory infection (35%), diarrhea (32%), headache (30%), anemia (24%), vomiting (22%), and nausea (19%). Twenty of 37 patients (54%) in the aHUS trials experienced a serious adverse event (SAE); the most commonly reported SAEs were hypertension (16%) and infections (14%).[8]
Eculizumab inhibits terminal complement activation and therefore makes patients vulnerable to infection with encapsulated organisms. Life-threatening and fatal meningococcal infections have occurred in patients who received eculizumab.[8] Clinicians are therefore advised to comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for meningococcal vaccination in patients with complement deficiencies. Due to the increased risk of meningococcal infections, meningococcal vaccination is recommended at least 2 weeks prior to receiving eculizumab, unless the risks of delaying eculizumab therapy outweigh the risk of developing a meningococcal infection. If urgent eculizumab therapy is indicated in an unvaccinated patient, the meningococcal vaccine should be administered as soon as possible.[8] However, current meningococcal vaccines do not protect against strains of meningococcus with a serogroup B antigen,[25] and thus may not be sufficient to protect patients. In clinical trials, 33 of 67 patients with aHUS were treated with eculizumab less than 2 weeks after meningococcal vaccination, and 31 of these 33 patients received antibiotics for prophylaxis of meningococcal infection until at least 2 weeks after vaccination. Patients should be monitored for early sign of meningococcal infection, and should be evaluated immediately if infection is suspected.[8]
Eculizumab treatment is recommended to continue for the patient’s lifetime, unless discontinuation of therapy is clinically indicated.[26] After discontinuing eculizumab, patients with PNH should be monitored for at least 8 weeks to detect hemolysis. Patients with aHUS should be monitored for signs and symptoms of TMA complications for at least 12 weeks. In aHUS clinical studies, 18 patients (five in the prospective studies) discontinued eculizumab treatment; TMA complications occurred following a missed dose in five patients, and eculizumab was reinstated in four of these five patients. If TMA complications occur after eculizumab discontinuation, the clinician should consider reinstitution of eculizumab treatment, PE/PI, or appropriate organ-specific supportive measures.[8]
Mechanism of Action
Eculizumab is a recombinant humanized monoclonal IgG2/4 antibody[27] that selectively targets and inhibits the terminal portion of the complement cascade. The complement system is a branch of the body’s immune system that destroys and removes foreign particles. When complement proteins are activated and bind to the surfaces of foreign particles, it triggers a cascade by which one complement protein induces the activation of the next protein in the sequence. The complement proteins then create holes or pores in the invading organisms, leading to their destruction. While complement plays an important role in protecting the body from foreign organisms, it can also destroy healthy cells and tissue.
Eculizumab specifically binds to the terminal Complement component 5, or C5, which acts at a late stage in the complement cascade. When activated, C5 is involved in activating host cells, thereby attracting pro-inflammatory immune cells, while also destroying cells by triggering pore formation. By inhibiting the complement cascade at this point, the normal, disease-preventing functions of proximal complement system are largely preserved, while the properties of C5 that promote inflammation and cell destruction are impeded.[28]
Eculizumab inhibits the cleavage of C5 to C5a (a potent anaphylatoxin with prothrombotic and proinflammatory properties) and C5b by the C5 convertase, which prevents the generation of the terminal complement complex C5b-9 (which also has prothrombotic and proinflammatory effects). Both C5a and C5b-9 cause the terminal complement-mediated events that are characteristic of PNH and aHUS.[28]
PNH
An acquired genetic mutation in patients with PNH leads to the generation from bone marrow of abnormal cell lines (known as PNH cells) that are deficient in protective complement inhibitors on the cell surface. PNH red blood cells undergo lysis due to constant attack by the body’s complement (immune) system. Eculizumab inhibits terminal complement-mediated chronic hemolysis in people with (PNH).[28]
aHUS
Eculizumab inhibits complement-mediated thrombotic microangiopathy (TMA) in patients with aHUS, a disease in which a deficiency of natural complement regulatory factors leads to chronic uncontrolled complement activation. This results in platelet activation, endothelialcell damage, and systemic, persistent TMA.[8]
Biochemistry
Eculizumab is a humanized monoclonal antibody against the complement protein C5. It is an immunoglobulin G-kappa (IgGκ) consisting of human constant regions and murine complementarity-determining regions grafted onto human framework light and heavy chain variable regions. The compound contains two 448-amino acid heavy chains and two 214-amino acid light chains, and has a molecular weight of approximately 148 kilodaltons (kDa).[29]
The metabolism of eculizumab is thought to occur via lysosomal enzymes that cleave the antibody to generate small peptides and amino acids. The volume of distribution of eculizumab in humans approximates that of plasma.[29]
Research
STEC-HUS
There are case reports of eculizumab being used to treat Shiga-toxin-producing Escherichia coli hemolytic-uremic syndrome (STEC-HUS),[30] such as occurred during the May 2011 outbreak of enteroaggretative E. coli infections in Germany (the STEC-HUS commonly seen in North America is of the enterohemorrhagic type). Eculizumab was given to block complement activation, which plays a role in the pathogenesis of both STEC-HUS and aHUS. Specifically, Shiga-toxin has been shown to trigger uncontrolled complement activity through direct activation of the alternative complement pathway[31][32] as well as by binding to and inactivating the regulatory protein complement factor H.[33]
Acute Humoral Rejection (AHR)/ Antibody-Mediated Rejection (AMR)
Preliminary results from a Mayo Clinic research study show that eculizumab prevents acute humoral rejection (AHR, also known as antibody-mediated rejection (AMR)) of kidney allografts by inhibiting activation of the complement system by antigen-antibody complexes.[34] Specifically, eculizumab treatment led to a significant decrease in the incidence of early AHR, compared to a historical control group that received no eculizumab. Eculizumab also reportedly maintained stable allograft function and simplified the management of kidney transplant patients by decreasing the need for post-transplant plasma exchange and splenectomy.[35] Researchers at Johns Hopkins University have also reported a case in which eculizumab was combined with plasmapheresis and intravenous immunoglobulin to salvage a kidney undergoing severe AMR. In this case, eculizumab, by inhibiting the cleavage of complement protein C5 to the C5a and C5b receptors, was associated with a marked decrease in membrane attack complex (C5b-9) deposition in the kidney.[36]
Myasthenia Gravis
Eculizumab has been shown to produce a clinically meaningful benefit in patients with severe and refractory generalized myasthenia gravis, a rare neurological disorder caused by uncontrolled complement activation resulting from auto-antibodies that recognize a specific target in the nerve-muscle junction.[37] In a Phase II study involving 14 patients, eculizumab was superior to placebo in improving disease severity scores, and the improvement was achieved more rapidly with eculizumab than with placebo.[38]
Neuromyelitis Optica
An open-label study is investigating the effects of eculizumab in patients with neuromyelitis optica, a complement-mediated inflammatory disease of the brain tissues that can potentiate immune attack on the optic nerves (leading to optic neuritis), spinal cord (causing transverse myelitis), and brain.[39]
Membranoproliferative Glumerulonephritis (MPGN)
Membranoproliferative glomerulonephritis (MPGN, previously known as mesangiocapillary glomerulonephritis) is an uncommon cause of chronic nephritis that primarily affects children but can occur at any age. Its clinical presentation and course can range from benign and slowly progressive to rapidly progressive. Patients may thus present with hematuria (blood in the urine), proteinuria (excess protein in the urine), renal impairment, and hypertension. MPGN frequently progresses to end-stage renal disease (ESRD) and disease recurrence following kidney transplantation.[40] Some cases of the disease are thought to result from complement dysregulation.[40][41][42][43] Canadian researchers have reported a case in which eculizumab produced “a dramatic response” in a 16-year-old girl with MPGN, as evidenced by amelioration of neurologic complications, normalization of kidney function, and improvements in thrombocytopenia, anemia, proteinuria, and hypoalbuminemia.[42][43]
Dense-Deposit Disease (DDD)
Dense-deposit disease (DDD), previously considered a subtype of MPGN (sometimes known as MPGN II), is characterized by dense deposits of immunoglobulins, complement factors, or both in the basement membrane of the glomerulus.[40] DDD frequently progresses to ESRD and disease recurrence after kidney transplantation.[44] In a March 22, 2012 letter to the New England Journal of Medicine, a group of Italian researchers reported a case involving an 11-year-old girl with DDD who was treated with eculizumab, which led to normalization of serum total protein and albumin, decreased creatinine, and decline of proteinuria to below the nephrotic range.[45] The same issue of the New England Journal of Medicine also featured a letter from another group of Italian researchers, who reported a case in which a 17-year-old patient with DDD experienced improvements in proteinuria, plasma protein levels, and renal function, along with reductions in the size of dense deposits, after treatment with eculizumab. After treatment was interrupted after 18 months, proteinuria rapidly increased; eculizumab therapy was resumed 6 months later, and was associated with a reduction in proteinuria.[44] In a Phase I trial involving three patients with DDD and three with C3 glomerulonephritis who were treated with eculizumab every other week for 1 year, two patients showed significantly reduced serum creatinine, one achieved a marked reduction in proteinuria, and one had stable laboratory parameters but histopathologic improvements. The investigators surmised that pre-treatment elevation of serum membrane attack complex may predict response to eculizumab in DDD and C3 glomerulonephritis, both of which comprise C3 glomerulopathy.[46]
Cold Agglutinin Disease
There are also reports of eculizumab being used to treat cold agglutinin disease. In one patient case report, eculizumab led to a sustained reduction of hemolysis, disappearance of further exacerbations, complete elimination of transfusion requirements, and improvement of symptoms and quality of life.[47]
Catastrophic Antiphospholipid Syndrome (CAPS)
Catastrophic antiphospholipid syndrome (CAPS) is a rare condition in which blood clots form in multiple organs simultaneously, possibly leading to multi-organ system failure and death. The kidneys are the most frequently affected organ system in CAPS, and patients who survive a CAPS episode commonly experience permanent kidney failure.[48] There are reports in the literature suggesting that eculizumab therapy may be useful in CAPS by virtue of its blockade of complement activity, prevention of acute progressive thrombotic events, reversal of thrombocytopenia, and control of serum antiphospholipid antibody levels.[49][50]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Hillmen, Young, Schubert, P, N, J; et al. (2006). "The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria". N Engl J Med. 355 (12): 1233–1243. doi:10.1056/NEJMMoa061648. PMID 16990386.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help);|first15=missing|last15=in Authors list (help);|first16=missing|last16=in Authors list (help);|first17=missing|last17=in Authors list (help);|first18=missing|last18=in Authors list (help);|first19=missing|last19=in Authors list (help);|first20=missing|last20=in Authors list (help) - ↑ http://www.medicinescomplete.com/mc/bnf/current/PHP5923-soliris.htm?q=soliris&t=search&ss=text&p=1#PHP5923-soliris
- ↑ 3.0 3.1 Noris, Caprioli, Bresin, M, J, E; et al. (2010). "Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype". Clin J Am Soc Nephrol. 5 (10): 1844–1859. doi:10.2215/CJN.02210310. PMC 2974386. PMID 20595690.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help);|first15=missing|last15=in Authors list (help);|first16=missing|last16=in Authors list (help);|first17=missing|last17=in Authors list (help);|first18=missing|last18=in Authors list (help) - ↑ 4.0 4.1 4.2 4.3 4.4 Caprioli, Noris, Brioschi, J, M, S; et al. (2006). "Genetics of HUS: the impact of MPC, CFH, and IF mutations on clinical presentation, response to treatment, and outcome". Blood. 108 (4): 1267–1279. doi:10.1182/blood-2005-10-007252. PMC 1895874. PMID 16621965.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help);|first15=missing|last15=in Authors list (help);|first16=missing|last16=in Authors list (help);|first17=missing|last17=in Authors list (help);|first18=missing|last18=in Authors list (help) - ↑ 5.0 5.1 5.2 Hillman, Hall, Marsh, P, C, JC; et al. (2004). "Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria". N Eng J Med. 350 (6): 552–559. doi:10.1056/NEJMoa031688.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help) - ↑ 6.0 6.1 Ray, Burrows, Ginsberg, Burrows, JG, RF, JS, EA (2000). "Paroxysmal nocturnal hemoglobinuria and the risk of venous thrombosis: review and recommendations for management of the pregnant and nonpregnant patient". Haemostasis. 30: 103–107.
- ↑ Kelly, Hill, Arnold, RJ, A, LM; et al. (2011). "Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival". Blood. 117 (25): 6786–6792. doi:10.1182/blood-2011-02-333997. PMID 21460245.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help) - ↑ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 8.12 8.13 8.14 "7.Soliris® (eculizumab) prescribing information" (PDF). Cheshire, CT: Alexion Pharmaceuticals. 2011. Retrieved 15 December 2013.
- ↑ Hillmen, Lewis, Bessler, Luzzatto, Dacie, P, SM, M, L, JV (1995). "Natural history of paroxysmal nocturnal hemoglobinuria". N Engl J Med. 333 (19): 1253–1258. doi:10.1056/NEJM199511093331904. PMID 7566002.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ 10.0 10.1 10.2 Hillmen, Muus, Duhrsen, P, P, U; et al. (2007). "Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria". Blood. 110 (12): 4123–4128. doi:10.1182/blood-2007-06-095646. PMID 17702897.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help);|first15=missing|last15=in Authors list (help);|first16=missing|last16=in Authors list (help) - ↑ Brodsky, RA; Hoffman R, Benz EJ Jr, Shattil SJ; et al. (2005). "Paroxysmal nocturnal hemoglobinuria". Hematology: Basic Principles and Practice. Philadelphia, PA: Elsevier Churchill Livingstone: 419–427.
- ↑ 12.0 12.1 Hill, Ridley, Esser, A, SH, D; et al. (2006). "Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: A new approach to PNH therapy". Blood. 107 (5): 2131–2137. doi:10.1182/blood-2005-02-0782. PMID 16322479.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help) - ↑ DeStafano, Rossi, Paciaroni, Leone, V, E, K, G (2002). "Screening for inherited thrombophilia: indications and therapeutic implications". Haematologica. 107 (5): 1095–1108.
- ↑ Hill, Richards, Hillmen, A, SJ, P (2007). "Recent developments in the understanding and management of paroxysmal nocturnal hemoglobinuria". Br J Haematol. 137 (3): 181–192. doi:10.1111/j.1365-2141.2007.06554.x. PMID 17408457.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help) - ↑ 15.0 15.1 Lee, Jang, Lee, JW, JH, JH; et al. (2010). "High prevalence and mortality associated with thromboembolism in Asian patients with paroxysmal nocturnal hemoglobinuria (PNH)". Haematologica. 95 (Suppl 2): 205, abstr.0505.
- ↑ Kim, Lee, Kim, Lee, Chung, JS, JW, BK, J (2010). "The use of the complement inhibitor eculizumab (Soliris) for treating Korean patients with paroxysmal nocturnal hemoglobinuria". Korean J Hematol. 45 (4): 269–74. doi:10.5045/kjh.2010.45.4.269. PMC 3023054. PMID 21253430.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ George, JN (2010). "How I treat patients with thrombotic thrombocytopenic purpura". Blood. 116 (20): 4060–4069. doi:10.1182/blood-2010-07-271445. PMID 20686117.
- ↑ Mache, Acham-Roschitz, Fremeaux-Bacchi, CJ, B, V; et al. (2009). "Complement inhibitor eculizumab in atypical hemolytic uremic syndrome". CJASN-- Cling J Amer Soc Nephrol. 4 (8): 1312–1316. doi:10.2215/CJN.01090209.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help) - ↑ 19.0 19.1 19.2 Licht, Muus, Legendre, C, P, C; et al. "Eculizumab is an effective long-term treatment in patients with atypical hemolytic-uremic syndrome (aHUS) previously receiving chronic plasma exchange/infusion (PE/PI): extenstion study results". Presented ath: 53rd Annual Society of Hematology Annual Meeting and Exposition. San Diego, CA: Poster TH- P0366.
December 10-13, 2011
- ↑ 20.0 20.1 Greenbaum, Babu, Furman, L, S, R; et al. "Continued improvements in renal function with sustained eculizumab in patients aith atypical hemolytic uremic syndrome (aHUS)". Presented at the American Society of Nephrology Kidney Week 2011. Philadelphia, PA: Pster TH-P0367.
November 8-13, 2011
- ↑ Legendre, Greenbaum, Babu, C, L, S; et al. "Eculizumab (ECU) is atypical hemolytic uremic syndrome (aHUS) patients (PTS) with progressing TMA: continued improvements at 2-year follow-up". Presented at: American Society of Nephrological Kidney Week 2012. San Diego, CA.
October 30-November 4, 2012
- ↑ Licht, Muus, Legendre, C, P, C; et al. "Eculizumab (ECU) is effective in atypical hemolytic uremic syndrome (aHUS) patients (PTS) with a long disease duration and chronic kidney disease (CKD): 2-year data". Presented at: American Society of Nephrology Kidney Week. San Diego, CA.
October 30-November 4, 2012
- ↑ Hodgkins, Bobrowski, Lane, Langman, KS, AE, JC, CB (2012). "Clinical grand rounds: atypical hemolytic uremic syndrome". Amer J Nephrol. 35 (5): 394–400. doi:10.1159/000337954.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help) - ↑ Gruppo, Dixon, RA, BP. "Long-term outcome in a pediatric patient with atypical hemolytic uremic syndrome (aHUS) with sustained eculizumab (ECU) treatment". Presented at: 53rd annual meeting and exposition of the american society of hematology. San Diego, CA: Abstract 44320.
December 10-13, 2011
- ↑ Bouts, Monnens, Davin, Struijk, Spanjaard, A, L, J-C, G, L (2011). "Insufficient protection by Neisseria meningitidis vaccination alone during eculizumab therpay". Pediatr Nephrol. 26 (10): 1919–1920. doi:10.1007/s00467-011-1929-3. PMC 3163808. PMID 21643943.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ "European Medicines Agency" (PDF). European Public Assessment Report (EPAR) for Soliris (eculizumab) Appendix I Summary of Product Characteristics. 2012.
- ↑ Rother, Rollins, Mojcik, RP, SA, CF; et al. (2007). "Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria". Nat Biotechnol. 25 (11): 1256–1264. doi:10.1038/nbt1344. PMID 17989688.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ 28.0 28.1 28.2 Brodsky, RA; Hoffman R, Benz EJ Jr, Shattil S; et al. (2009). "Paroxysmal nocturnal hemoglobinuria". Hematology: Basic Principles and Practice. Philadelphia, PA: Churchill Livingstone: 385–395.
- ↑ 29.0 29.1 Dmytrijuk, Robie-Suh, Cohen, Rieves, Weiss, Pazdur, A, K, MH, D, K, R (2008). "FDA report eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria". The Oncologist. 13 (9): 993–1000. doi:10.1634/theoncologist.2008-0086. PMID 18784156.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help) - ↑ Lapeyraque, Malina, Fremeaux-Bacchi, A-L, M, V; et al. (2011). "Eculizumab in Severe Shiga-Toxin- Associated HUS". N Engl J Med. 364 (26): 2561–2563. doi:10.1056/NEJMc1100859. PMID 21612462.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help) - ↑ Morigi, Galbusera, Gastoldi, M, M, S; et al. (2011). "Alternative pathway activation of complement by shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis". J Immunol. 187 (1): 172–180. doi:10.4049/jimmunol.1100491. PMID 21642543.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help) - ↑ Thurman, Marians, Emlen, JM, R, W; et al. (2009). "Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome". Clin J Am Soc Nephrol. 4 (12): 1920–1924. doi:10.2215/CJN.02730409. PMC 2798880. PMID 19820137.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help) - ↑ Orth, Khan, Naim, D, AB, A; et al. (2009). "Shiga toxin activated complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome". J Immunol. 182 (10): 6394–6400. doi:10.4049/jimmunol.0900151. PMID 19414792.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help) - ↑ "Study Should New Approach to Prevent Antibody-Mediated Damage in Kidney Transplants". Mayo Clinic (press release). June 2, 2009.
- ↑ Stegall, Diwan, Cornell, MD, TS, L; et al. (2010). "Terminal complement inhibition decreases early acute humoral rejection in sensitized renal transplant patientes". Trasplantation. 90: 127. doi:10.1097/00007890-201007272-00246.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help) - ↑ Locke, Magro, Singer, JE, CM, AL; et al. (2009). "The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection". Am J Transplant. 9 (1): 231–235. doi:10.1111/j.1600-6143.2008.02451.x. PMID 18976298.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help);|first13=missing|last13=in Authors list (help);|first14=missing|last14=in Authors list (help) - ↑ Complement associated pathogenic mechanisms in myasthenia gravis, Erdem Tüzün et al, 2013
- ↑ "34. Phase 2 study of eculizumab (Soliris®) in patients with severe and refractory generalized myasthenia gravis presented at MGFR annual meeting". Alexion Pharmaceuticals Inc. (press release). Cheshire, CT. September 14, 2011. horizontal tab character in
|title=at position 4 (help) - ↑ "An open label study of the effects of eculizumab in neuromyelitis optica." Bethesda, MD; National Institutes of Health, US Department of Health and Human Services". ClinicalTrials.gov. 2012.
- ↑ 40.0 40.1 40.2 Sethi, Fervenza, S, FC (2012). "Membranoproliferative glomerulonephritis - a new look at an old entity". N Engl J Med. 366 (12): 1119–1131. doi:10.1056/NEJMra1108178. PMID 22435371.
|first2=missing|last2=in Authors list (help) - ↑ Merk & Co., Inc. "Nephrotic syndrome". The Merk Manual Home Health Handbook. Whitehouse station, NJ.
- ↑ 42.0 42.1 Licht, Fremeaux-Bacchi, C, V (2009). "Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis". Thromb Haemost. 101 (2): 271–278. PMID 19190809.
|first2=missing|last2=in Authors list (help) - ↑ 43.0 43.1 Radhakrishnan, Lunn, Kirschfink, S, A, M; et al. (2012). "Eculizumab and refractory membranoproliferative glomerulonephritis". N Eng J Med. 366 (12): 1165–1166. doi:10.1056/NEJMc1106619.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help) - ↑ 44.0 44.1 Vivarelli, Pasini, Emma, M, A, F (2012). "Eculizumab for the treatment of dense-deposit disease". N Engl J Med. 366 (12): 1161–1165. doi:10.1056/NEJMc1111953.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help) - ↑ Daina, Noris, Remuzzi, E, M, G (2012). "Eculizumab in a patient with dense-deposit disease". N Engl J Med. 366 (12): 1161–1163. doi:10.1056/NEJMc1112273. PMID 22435382.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help) - ↑ Bomback, Smith, Barile, AS, RJ, GR (2012). "Eculizumab for dense deposit disease and C3 glomerulonephritis". Clin J Am Soc Nephrol. 5. 7 (5): 748–756. doi:10.2215/CJN.12901211. PMC 3338285. PMID 22403278.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help);|first7=missing|last7=in Authors list (help);|first8=missing|last8=in Authors list (help);|first9=missing|last9=in Authors list (help);|first10=missing|last10=in Authors list (help);|first11=missing|last11=in Authors list (help);|first12=missing|last12=in Authors list (help) - ↑ Roth, Huttmann, Rother, Duhrsen, Philipp, A, A, RP, U, T (2009). "Long-term efficacy of the complement inhibitor eculizumab in cold agglutinin disease". Blood. 113 (16): 3885–3886. doi:10.1182/blood-2009-01-196329. PMID 19372265.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help) - ↑ National Institutes of Health, US Department of Health and Human Services (2011). "Eculizumab to enable renal transplantation in patient with history of catastrophic antipholopid antibody syndrome". ClinicalTrials.gov. Bethesda, MD.
- ↑ Lonze, Singer, Montgomery, BE, AL, RA (2010). "Eculizumab and renal transplantation in a patient with CAPS". N Engl J Med. 362 (18): 1744–1745. doi:10.1056/NEJMc0910965. PMID 20445191.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help) - ↑ Shapira, Andrade, Allen, Salmon, I, D, SL, JE (2012). "Induction of durable remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab". Arthritis Rheum. 64 (8): 2719–2723. doi:10.1002/art.34440. PMID 22354668.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help)
External Links
- Pages with script errors
- CS1 errors: missing author or editor
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- CS1 errors: invisible characters
- Template:drugs.com link with non-standard subpage
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without KEGG source
- Articles without InChI source
- Drug has EMA link
- Drugboxes which contain changes to verified fields
- Infobox drug tracked parameters
- Drugs that are a monoclonal antibody
- Drugs