Ebastine: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 50: | Line 50: | ||
| StdInChIKey = MJJALKDDGIKVBE-UHFFFAOYSA-N | | StdInChIKey = MJJALKDDGIKVBE-UHFFFAOYSA-N | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Ebastine''' (trade names '''Evastin''', '''Kestine''', '''Ebastel''', '''Aleva''', '''Ebatrol''') is a H<sub>1</sub> [[antihistamine]] with low potential for causing drowsiness. | '''Ebastine''' (trade names '''Evastin''', '''Kestine''', '''Ebastel''', '''Aleva''', '''Ebatrol''') is a H<sub>1</sub> [[antihistamine]] with low potential for causing drowsiness. | ||
It does not penetrate the [[blood–brain barrier]] to a significant amount and thus combines an effective block of the [[histamine H1 receptor|H<sub>1</sub> receptor]] in peripheral [[biological tissue|tissue]] with a low incidence of central side effects, i.e. seldom causing sedation or drowsiness.<ref name="Tagawa 2001" /><ref name="Arzneistoff-Profile" /><ref name="Bousquet 1999" /> | It does not penetrate the [[blood–brain barrier]] to a significant amount and thus combines an effective block of the [[histamine H1 receptor|H<sub>1</sub> receptor]] in peripheral [[biological tissue|tissue]] with a low incidence of central side effects, i.e. seldom causing sedation or drowsiness.<ref name="Tagawa 2001">{{cite journal|last=Tagawa |first=M |last2=Kano |first2=M |last3=Okamura |first3=N |title=Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, and (+)-clorphrniramine, a classical antihistamine |journal=[[Br J Clin Pharmacol]] |year=2001 |volume=52 |issue=5 |pages=501–509 |pmc=2014616 |pmid=11736858 |doi=10.1046/j.1365-2125.2001.01471.x}} </ref><ref name="Arzneistoff-Profile">{{cite book|title=Arzneistoff-Profile|editor=Dinnendahl, V, Fricke, U|publisher=Govi Pharmazeutischer Verlag|location=Eschborn, Germany|year=2010|edition=23|volume=4|isbn=978-3-7741-98-46-3|language=German}}</ref><ref name="Bousquet 1999">{{cite journal|last=Bousquet |first=J |last2=Gaudaño |first2=EM |last3=Palama Carlos |first3=AG |title=A 12-week, placebo-controlled study of the efficacy and safety of ebastine, 10 and 20 mg once daily, in the treatment of perennial allergic rhinitis |journal=Allergy |year=1999 |volume=54 |issue=6 |pages=562–568 |pmid=10435469 |doi=10.1034/j.1398-9995.1999.00984.x}}</ref> | ||
The patent in which the structure of ebastine is first mentioned is {{patent|EP|134124}} in Europe and {{patent|US|4550116}} in the US. The substance is often provided in [[micronise]]d form due to poor water solubility. | The patent in which the structure of ebastine is first mentioned is {{patent|EP|134124}} in Europe and {{patent|US|4550116}} in the US. The substance is often provided in [[micronise]]d form due to poor water solubility. | ||
==Uses and availability== | ==Uses and availability== | ||
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic [[rhinitis]] and chronic idiopathic [[urticaria]].<ref name="Van Cauwenberge 2004" /> It is available in 10 and 20 mg tablets<ref name="Sastre 2008" /> and as fast-dissolving tablets,<ref name="pmid 17697901" /> as well as in pediatric syrup. It has a recommended flexible daily dose of 10 or 20 mg, depending on disease severity. | Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic [[rhinitis]] and chronic idiopathic [[urticaria]].<ref name="Van Cauwenberge 2004">{{cite journal|last=Van Cauwenberge |first=P |last2=de Belder |first2=T |last3=Sys |first3=L |title=A review of the second-generation antihistamine ebastine for the treatment of allergic disorders |journal=Exp Rew Pharmacother |year=2004 |volume=5 |issue=8 |pages=1807–13 |pmid=15264995 |doi=10.1517/14656566.5.8.1807}}</ref> It is available in 10 and 20 mg tablets<ref name="Sastre 2008">{{cite journal|last=Sastre |first=J |title=Ebastine in allergic rhinitis and chronic idiopathic urticaria |journal=Allergy |year=2008 |volume=63 |issue=Suppl 89 |pages=1–20 |doi=10.1111/j.1398-9995.2008.01897.x |pmid=19032340}}</ref> and as fast-dissolving tablets,<ref name="pmid 17697901">{{cite journal|last=Antonijoan |first=R |last2=García-Gea |first2=C |last3=Puntes |first3=M |title=Comparison of inhibition of cutaneous histamine reaction of ebastine fast-dissolving tablet (20 mg) versus desloratadine capsules (5 mg): a randomized, double-blind, double-dummy, placebo-controlled, three period crossover study in healthy, nonatopic adults |journal=Clin Ther |year=2007 |volume=29 |issue=5 |pages=814–22 |pmid=17697901 |doi=10.1016/j.clinthera.2007.05.001}}</ref> as well as in pediatric syrup. It has a recommended flexible daily dose of 10 or 20 mg, depending on disease severity. | ||
Ebastine is available in different formulations (tablets, fast dissolving tablets and syrup) and commercialized under different brand names around the world, '''Ebet''', '''Ebastel FLAS''', '''Kestine''', '''KestineLIO''', '''KestinLYO''', '''EstivanLYO''', '''Evastel Z''', '''Ebatrol''', etc. | Ebastine is available in different formulations (tablets, fast dissolving tablets and syrup) and commercialized under different brand names around the world, '''Ebet''', '''Ebastel FLAS''', '''Kestine''', '''KestineLIO''', '''KestinLYO''', '''EstivanLYO''', '''Evastel Z''', '''Ebatrol''', etc. | ||
| Line 65: | Line 68: | ||
After oral administration, ebastine undergoes extensive [[first-pass metabolism]] by hepatic [[cytochrome P450 3A4]] into its active [[carboxylic acid]] metabolite, carebastine. This conversion is practically complete. | After oral administration, ebastine undergoes extensive [[first-pass metabolism]] by hepatic [[cytochrome P450 3A4]] into its active [[carboxylic acid]] metabolite, carebastine. This conversion is practically complete. | ||
[[File:Ebastine PK.png|none|left]] | [[File:Ebastine PK.png|none|left]] | ||
==Efficacy== | ==Efficacy== | ||
Data from over 8,000 patients in more than 40 clinical trials{{failed verification|date=March 2014}} and studies<ref name="Bousquet 1999" /><ref name="Van Cauwenberge 2004" /><ref name="Sastre 2008" /><ref name="Ratner 2005" /><ref name="pmid 17563125" /><ref name="Gehanno 1996" /> suggest efficacy of ebastine in the treatment of intermittent [[allergic rhinitis]], persistent allergic rhinitis and other indications. | Data from over 8,000 patients in more than 40 clinical trials{{failed verification|date=March 2014}} and studies<ref name="Bousquet 1999" /><ref name="Van Cauwenberge 2004" /><ref name="Sastre 2008" /><ref name="Ratner 2005">{{cite journal|last=Ratner |first=P |last2=Falqués |first2=M |last3=Chuecos |first3=F |title=Meta-analysis of the efficacy of ebastine 20 mg compared to loratadine 10 mg and placebo in the symptomatic treatment of seasonal allergic rhinitis |journal=Int Arch Allergy Immunol |year=2005 |volume=138 |issue=4 |pages=312–8 |pmid=16224195 |doi=10.1159/000088869}}</ref><ref name="pmid 17563125">{{cite journal|last=Antonijoan |first=RM |last2=García-Gea |first2=C |last3=Puntes |first3=M |title=A comparison of ebastine 10 mg fast-dissolving tablet with oral desloratadine and placebo in inhibiting the cutaneous reaction to histamine in healthy adults |journal=Clin Drug Invest |year=2007 |volume=27 |issue=7 |pages=453–61 |pmid=17563125 |doi=10.2165/00044011-200727070-00002}}</ref><ref name="Gehanno 1996">{{cite journal|last=Gehanno |first=P |last2=Bremard-Oury |first2=C |last3=Zeisser |first3=P |title=Comparison of ebastine to cetirizine in seasonal allergic rhinitis in adults |journal=Annals of Allergy, Asthma and Immunol |year=1996 |volume=76 |issue=6 |pages=507–12 |pmid=8673684 |doi=10.1016/S1081-1206(10)63269-3}}</ref> suggest efficacy of ebastine in the treatment of intermittent [[allergic rhinitis]], persistent allergic rhinitis and other indications. | ||
==Safety== | ==Safety== | ||
| Line 76: | Line 78: | ||
==References== | ==References== | ||
{{reflist|2 | {{reflist|2}} | ||
==External links== | ==External links== | ||
| Line 108: | Line 86: | ||
{{Histaminergics}} | {{Histaminergics}} | ||
[[Category:Drug]] | |||
[[Category:H1 receptor antagonists]] | [[Category:H1 receptor antagonists]] | ||
[[Category:Ethers]] | [[Category:Ethers]] | ||
[[Category:Piperidines]] | [[Category:Piperidines]] | ||
[[Category:Aromatic ketones]] | [[Category:Aromatic ketones]] | ||
Latest revision as of 18:12, 7 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | Greater than 95% |
| Metabolism | Hepatic (CYP3A4-mediated), to carebastine |
| Elimination half-life | 15 to 19 hours (carebastine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C32H39NO2 |
| Molar mass | 469.658 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Ebastine |
|
Articles |
|---|
|
Most recent articles on Ebastine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Ebastine at Clinical Trials.gov Clinical Trials on Ebastine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Ebastine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Ebastine Risk calculators and risk factors for Ebastine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Ebastine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Ebastine (trade names Evastin, Kestine, Ebastel, Aleva, Ebatrol) is a H1 antihistamine with low potential for causing drowsiness.
It does not penetrate the blood–brain barrier to a significant amount and thus combines an effective block of the H1 receptor in peripheral tissue with a low incidence of central side effects, i.e. seldom causing sedation or drowsiness.[1][2][3]
The patent in which the structure of ebastine is first mentioned is Template:Patent in Europe and Template:Patent in the US. The substance is often provided in micronised form due to poor water solubility.
Uses and availability
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic rhinitis and chronic idiopathic urticaria.[4] It is available in 10 and 20 mg tablets[5] and as fast-dissolving tablets,[6] as well as in pediatric syrup. It has a recommended flexible daily dose of 10 or 20 mg, depending on disease severity.
Ebastine is available in different formulations (tablets, fast dissolving tablets and syrup) and commercialized under different brand names around the world, Ebet, Ebastel FLAS, Kestine, KestineLIO, KestinLYO, EstivanLYO, Evastel Z, Ebatrol, etc.
Pharmacokinetic profile
After oral administration, ebastine undergoes extensive first-pass metabolism by hepatic cytochrome P450 3A4 into its active carboxylic acid metabolite, carebastine. This conversion is practically complete.
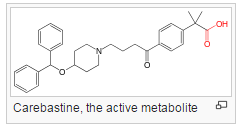
Efficacy
Data from over 8,000 patients in more than 40 clinical trials[not in citation given] and studies[3][4][5][7][8][9] suggest efficacy of ebastine in the treatment of intermittent allergic rhinitis, persistent allergic rhinitis and other indications.
Safety
Ebastine has shown overall safety and tolerability profile with no cognitive/psychomotor impairment[5] and no sedation[5] worse than placebo,[2] and cardiac safety, that is, no QT prolongation.[5] The incidence of most commonly reported adverse events was comparable between the ebastine and placebo groups, which confirms that ebastine has a favourable safety profile.
While experiments in pregnant animals showed no risk for the unborn, no such data are available in humans. It is not known whether ebastine passes into the breast milk.[2]
References
- ↑ Tagawa, M; Kano, M; Okamura, N (2001). "Neuroimaging of histamine H1-receptor occupancy in human brain by positron emission tomography (PET): a comparative study of ebastine, a second-generation antihistamine, and (+)-clorphrniramine, a classical antihistamine". Br J Clin Pharmacol. 52 (5): 501–509. doi:10.1046/j.1365-2125.2001.01471.x. PMC 2014616. PMID 11736858.
- ↑ 2.0 2.1 2.2 Dinnendahl, V, Fricke, U, ed. (2010). Arzneistoff-Profile (in German). 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-98-46-3.
- ↑ 3.0 3.1 Bousquet, J; Gaudaño, EM; Palama Carlos, AG (1999). "A 12-week, placebo-controlled study of the efficacy and safety of ebastine, 10 and 20 mg once daily, in the treatment of perennial allergic rhinitis". Allergy. 54 (6): 562–568. doi:10.1034/j.1398-9995.1999.00984.x. PMID 10435469.
- ↑ 4.0 4.1 Van Cauwenberge, P; de Belder, T; Sys, L (2004). "A review of the second-generation antihistamine ebastine for the treatment of allergic disorders". Exp Rew Pharmacother. 5 (8): 1807–13. doi:10.1517/14656566.5.8.1807. PMID 15264995.
- ↑ 5.0 5.1 5.2 5.3 5.4 Sastre, J (2008). "Ebastine in allergic rhinitis and chronic idiopathic urticaria". Allergy. 63 (Suppl 89): 1–20. doi:10.1111/j.1398-9995.2008.01897.x. PMID 19032340.
- ↑ Antonijoan, R; García-Gea, C; Puntes, M (2007). "Comparison of inhibition of cutaneous histamine reaction of ebastine fast-dissolving tablet (20 mg) versus desloratadine capsules (5 mg): a randomized, double-blind, double-dummy, placebo-controlled, three period crossover study in healthy, nonatopic adults". Clin Ther. 29 (5): 814–22. doi:10.1016/j.clinthera.2007.05.001. PMID 17697901.
- ↑ Ratner, P; Falqués, M; Chuecos, F (2005). "Meta-analysis of the efficacy of ebastine 20 mg compared to loratadine 10 mg and placebo in the symptomatic treatment of seasonal allergic rhinitis". Int Arch Allergy Immunol. 138 (4): 312–8. doi:10.1159/000088869. PMID 16224195.
- ↑ Antonijoan, RM; García-Gea, C; Puntes, M (2007). "A comparison of ebastine 10 mg fast-dissolving tablet with oral desloratadine and placebo in inhibiting the cutaneous reaction to histamine in healthy adults". Clin Drug Invest. 27 (7): 453–61. doi:10.2165/00044011-200727070-00002. PMID 17563125.
- ↑ Gehanno, P; Bremard-Oury, C; Zeisser, P (1996). "Comparison of ebastine to cetirizine in seasonal allergic rhinitis in adults". Annals of Allergy, Asthma and Immunol. 76 (6): 507–12. doi:10.1016/S1081-1206(10)63269-3. PMID 8673684.
External links
- "KESTINE Package Insert". South African Electronic Package Inserts. 1997-10-24. Retrieved 2007-04-01.
- Pages with script errors
- CS1 maint: Multiple names: editors list
- CS1 maint: Unrecognized language
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- All articles with failed verification
- Articles with failed verification from March 2014
- Articles with invalid date parameter in template
- Drug
- H1 receptor antagonists
- Ethers
- Piperidines
- Aromatic ketones