Drug induced liver injury pathophysiology
Template:Drug-induced hepatitis Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Pathophysiology
The pathophysiologic mechanisms of hepatotoxicity are still being explored and include both hepatocellular and extracellular mechanisms. The following are some of the mechanisms that have been described:
- Apoptosis of hepatocytes: Activation of the apoptotic pathways by the tumor necrosis factor-alpha receptor of Fas may trigger the cascade of intercellular caspases, which results in programmed cell death.
- Bile duct injury: Toxic metabolites excreted in bile may cause injury to the bile duct epithelium.
- Cytolytic T-cell activation: Covalent binding of a drug to the P450 enzyme acts as an immunogen, activating T cells and cytokines and stimulating a multifaceted immune response.
- Disruption of the hepatocyte: Covalent binding of the drug to intracellular proteins can cause a decrease in ATP levels, leading to actin disruption. Disassembly of actin fibrils at the surface of the hepatocyte causes blebs and rupture of the membrane.
- Disruption of the transport proteins: Drugs that affect transport proteins at the canalicular membrane can interrupt bile flow. Loss of villous processes and interruption of transport pumps such as multidrug resistance–associated protein 3 prevent the excretion of bilirubin, causing cholestasis.
- Mitochondrial disruption: Certain drugs inhibit mitochondrial function by a dual effect on both beta-oxidation energy production by inhibiting the synthesis of nicotinamide adenine dinucleotide and flavin adenine dinucleotide, resulting in decreased ATP production.
The classic division of drug reactions is into at least 2 major groups:
- Drugs that directly affect the liver
- Drugs that mediate an immune response.
Drug Toxicity Mechanisms
- Hypersensitivity: Phenytoin is a classic, if not common, cause of hypersensitivity reactions. The response is characterized by fever, rash, and eosinophilia and is an immune-related response with a typical short latency period of 1-4 weeks.
- Idiosyncratic Drug Reactions: Idiosyncratic drug reactions can be subdivided into those that are classified as hypersensitivity or immunoallergic and those that are metabolic-idiosyncratic.
- Intrinsic or predictable drug reactions: Drugs that fall into this category cause reproducible injuries in animals, and the injury is dose related. The *injury can be due to the drug itself or to a metabolite. Acetaminophen is a classic example of a known intrinsic or predictable hepatotoxin at supertherapeutic doses. Another classic example is carbon tetrachloride.
- Metabolic-idiosyncratic: This type of reaction occurs through an indirect metabolite of the offending drug. Unlike intrinsic hepatotoxins, the response rate is variable and can occur within a week or up to one year later. It occurs in a minority of patients taking the drug, and no clinical manifestations of hypersensitivity are noted. INH toxicity is considered to fall into this class. Not all drugs fall neatly into one of these categories, and overlapping mechanisms may occur with some drugs (e.g., halothane).
Drug metabolism in liver
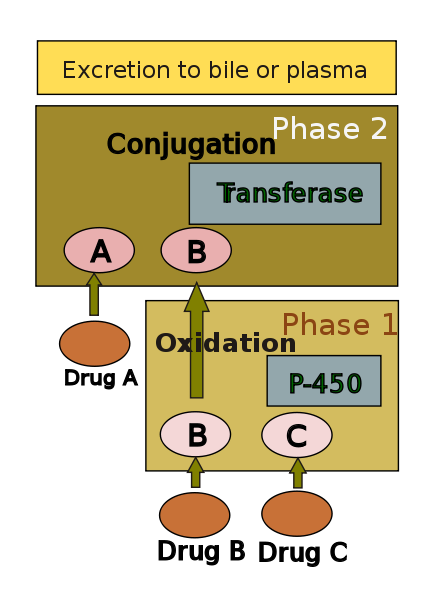
The human body identifies almost all drugs as foreign substances (i.e. xenobiotics) and subjects them to various chemical processes (i.e. metabolism) to make them suitable for elimination. This involves chemical transformations to (a) reduce fat solubility and (b) to change biological activity. Although almost all tissue in the body have some ability to metabolize chemicals, smooth endoplasmic reticulum in liver is the principal "metabolic clearing house" for both endogenous chemicals (e.g., cholesterol, steroid hormones, fatty acids, and proteins), and exogenous substances (e.g. drugs).[1] The central role played by liver in the clearance and transformation of chemicals also makes it susceptible to drug induced injury.
Drug metabolism is usually divided into two phases: phase 1 and phase 2. Phase 1 reaction is thought to prepare a drug for phase 2. However many compounds can be metabolised by phase 2 directly. Phase 1 reaction involves oxidation, reduction, hydrolysis, hydration and many other rare chemical reactions. These processes tend to increase water solubility of the drug and can generate metabolites which are more chemically active and potentially toxic. Most of phase 2 reactions take place in cytosol and involve conjugation with endogenous compounds via transferase enzymes. Chemically active phase 1 products are rendered relatively inert and suitable for elimination by this step.
A group of enzymes located in the endoplasmic reticulum, known as cytochrome P-450, is the most important family of metabolizing enzymes in the liver. Cytochrome P-450 is the terminal oxidase component of an electron transport chain. It is not a single enzyme, rather consists of a family of closely related 50 isoforms, six of them metabolize 90% of drugs.[2][3] There is a tremendous diversity of individual P-450 gene products and this heterogeneity allows the liver to perform oxidation on a vast array of chemicals (including almost all drugs) in phase 1. Three important characteristics of the P450 system have roles in drug induced toxicity:
- 1. Genetic diversity:
Each of the P-450 proteins is unique and accounts to some extent for the variation in drug metabolism between individuals. Genetic variations (polymorphism) in CYP450 metabolism should be considered when patients exhibit unusual sensitivity or resistance to drug effects at normal doses. Such polymorphism is also responsible for variable drug response among patients of differing ethnic backgrounds.
| Potent inducers | Potent inhibitors | Substrates |
|---|---|---|
| Rifampicin, Carbamazepine, Phenobarbital, Phenytoin, (St John's wort), |
Amiodarone, cimetidine, ciprofloxacin, fluconazole, fluoxetine, erythromycin, isoniazid, diltiazem |
Caffeine, clozapine, omeprazole, losartan, theophylline |
- 2. Change in enzyme activity:
Many substances can influence P-450 enzyme mechanism. Drugs interact with the enzyme family in several ways.[6] Drugs that modify Cytochrome P-450 enzyme are referred to as either inhibitors or inducers. Enzyme inhibitors block the metabolic activity of one or several P-450 enzymes. This effect usually occurs immediately. On the other hand inducers increase P-450 activity by increasing its synthesis. Depending on inducing drug's half life, there is usually a delay before enzyme activity increases.[3]
- 3. Competitive inhibition:
Some drugs may share the same P-450 specificity and thus competitively block their bio transformation. This may lead to accumulation of drugs metabolised by the enzyme. This type of drug interaction may also reduce the rate of generation of toxic substrate.
References
- ↑ Donald Blumenthal; Laurence Brunton; Keith Parker; Lazo, John S.; Iain Buxton. Goodman and Gilman's Pharmacological Basis of Therapeutics Digital Edition. McGraw-Hill Professional. ISBN 0-07-146804-8.
- ↑ Skett, Paul; Gibson, G. Gordon (2001). Introduction to drug metabolism. Cheltenham, UK: Nelson Thornes Publishers. ISBN 0-7487-6011-3.
- ↑ 3.0 3.1 3.2 Lynch T, Price A (2007). "The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects". American family physician. 76 (3): 391–6. PMID 17708140.
- ↑ Jessica R. Oesterheld; Kelly L. Cozza; Armstrong, Scott. Concise Guide to Drug Interaction Principles for Medical Practice: Cytochrome P450s, Ugts, P-Glycoproteins. Washington, DC: American Psychiatric Association. pp. 167–396. ISBN 1-58562-111-0.
- ↑ Flockhart DA. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine (2007). http://medicine.iupui.edu/flockhart/table.htm. Accessed [29-09-2007]
- ↑ Michalets EL (1998). "Update: clinically significant cytochrome P-450 drug interactions". Pharmacotherapy. 18 (1): 84–112. PMID 9469685.