Diabetes mellitus type 1 medical therapy: Difference between revisions
No edit summary |
|||
| (23 intermediate revisions by 4 users not shown) | |||
| Line 3: | Line 3: | ||
{{Diabetes mellitus}} | {{Diabetes mellitus}} | ||
{{CMG}}; {{AE}} [[Priyamvada Singh|Priyamvada Singh, M.B.B.S.]] [mailto:psingh13579@gmail.com]; {{CZ}} | {{CMG}}; {{AE}} [[Priyamvada Singh|Priyamvada Singh, M.B.B.S.]] [mailto:psingh13579@gmail.com]; {{CZ}}{{VD}} | ||
==Overview== | |||
See also: [[Blood glucose monitoring]]. | |||
The goals of [[therapy]] for [[diabetes mellitus type 1|type 1]] or [[diabetes mellitus type 2|type 2 diabetes mellitus]] ([[diabetes mellitus|DM]]) are to eliminate [[symptom|symptoms]] related to [[hyperglycemia]], reduce or eliminate the long-term microvascular and macrovascular [[Complication (medicine)|complications]] of [[diabetes mellitus|DM]], and allow the [[patient]] to achieve as normal lifestyle as possible. [[diabetes mellitus type 1|Type 1 diabetes]] is characterized by an absolute [[insulin]] deficiency. For these [[patient|patients]], a basal-bolus regimen with a long-acting [[Analog (chemistry)|analog]] and a short- or rapid-acting [[insulin]] [[Analog (chemistry)|analog]] is the most physiologic [[insulin]] regimen and the best option for optimal [[blood sugar|glycemic]] control. | |||
==Medical Therapy== | ==Medical Therapy== | ||
[[diabetes mellitus type 1|Type 1 diabetes]] is characterized by an absolute [[insulin]] deficiency. For these [[patient|patients]], a basal-bolus regimen with a long-acting [[Analog (chemistry)|analog]] and a short- or rapid-acting [[insulin]] [[Analog (chemistry)|analog]] is the most physiologic [[insulin]] regimen and the best option for optimal [[blood sugar|glycemic]] control. The medical [[therapy]] for [[diabetes mellitus type 1|type 1 DM]]:<ref>Type 1 Diabetes mellitus "Dennis Kasper, Anthony Fauci, Stephen Hauser, Dan Longo, J. Larry Jameson, Joseph Loscalzo"Harrison's Principles of Internal Medicine, 19e Accessed on December 27th,2016</ref><ref name="pmid27974167">{{cite journal| author=Strich D, Balagour L, Shenker J, Gillis D| title=Lower Basal Insulin Dose is Associated with Better Control in Type 1 Diabetes. | journal=J Pediatr | year= 2016 | volume= | issue= | pages= | pmid=27974167 | doi=10.1016/j.jpeds.2016.11.029 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27974167 }}</ref><ref>{{Cite web|url=http://www.tdctoolkit.org/wp-content/themes/tdc/algorithms/13_InsulinAlgorithmType1.pdf|title=Insulin treatment algorithms|last=|first=|date=|website=|publisher=|access-date=}}</ref> | |||
The | {| class="wikitable" | ||
* | ! colspan="4" |Duration of Action of Standard [[Insulin]] and [[Insulin]] [[Analog (chemistry)|Analogues]].* | ||
|- | |||
* | |'''[[Insulin]]''' | ||
|'''Onset of Action''' | |||
|'''Peak Action''' | |||
|'''Effective Duration''' | |||
|- | |||
| colspan="4" |'''Standard''' | |||
|- | |||
|[[insulin|Regular]] | |||
|30-60 min | |||
|2-3 hr | |||
|8-10 hr | |||
|- | |||
|[[insulin|NPH]] | |||
|2-4 hr | |||
|4-10 hr | |||
|12-18 hr | |||
|- | |||
|[[insulin|Zinc insulin(Lente)]] | |||
|2-4 hr | |||
|4-12 hr | |||
|12-20 hr | |||
|- | |||
|[[insulin|Extended Zinc insulin]] | |||
|6-10 hr | |||
|10-16 hr | |||
|18-24 hr | |||
|- | |||
| colspan="4" |'''Analogues''' | |||
|- | |||
|[[Insulin lispro|Lispro]] | |||
|5-15 min | |||
|30-90 min | |||
|4-6 hr | |||
|- | |||
|[[Insulin aspart|Aspart]] | |||
|5-15 min | |||
|30-90 min | |||
|4-6 hr | |||
|- | |||
|[[Insulin glargine|Glargine]] | |||
|2-4 hr | |||
|None | |||
|20-24 hr | |||
|- | |||
| colspan="4" |* Serum [[insulin]] profiles are based on a [[Subcutaneous tissue|subcutaneous]] [[Injection (medicine)|injection]] of 0.1 to 0.2 unit per kilogram of body weight; large variation within and between persons may be noted. Data are from DeWitt and Hirsch.6 | |||
|} | |||
== | {| class="wikitable" | ||
! colspan="3" |[[Insulin]] Algorithm for [[diabetes mellitus type 1|Type 1 Diabetes Mellitus]] in Children and Adults | |||
|- | |||
! colspan="3" |ABBREVIATIONS BASAL: [[Insulin glargine|Glargine]] or [[Insulin detemir|Detemir]] BOLUS (Prandial): Reg: [[insulin|Regular Insulin]] (peak action 3-4 hrs) RAI: Rapid Acting [[Insulin]] = [[Insulin aspart|Aspart]], [[Insulin glulisine|Glulisine]], or [[Insulin lispro|Lispro]] (peak action 1-1 ½ hrs) PPG: Post-Prandial [[Glucose]] SMBG: Self-monitored [[blood sugar|blood glucose]] TDI: Total daily [[insulin]] dosage in units | |||
|- | |||
|Split-Mix [[Insulin]] [[therapy|Therapies]] | |||
|1. Two shots: [[insulin|NPH]] + [[insulin|Reg]] or RAI 2:1 ratio AM; 1:1 ratio PM | |||
2. Three shots: AM: NPH + Reg or RAI | |||
PM: [[insulin|Reg]] or RAI | |||
HS: [[insulin|NPH]] | |||
2/3 TDI ÷ as 2/3 AM [[insulin|NPH]] + 1/3 as [[insulin|Reg]] or RAI | |||
1/3 TDI ÷ as ½ PM [[insulin|Reg]] or RAI + ½ [[insulin|NPH]] at HS | |||
3. Two shots Premix 2/3 AM + 1/3 PM | |||
Total Daily [[Insulin]] : 0.3-0.5 units/kg/day, and titrate to glycemic targets | |||
|Follow [[Glycosylated hemoglobin|A1c]] Every 3-6 months and Adjust Regimen to Maintain [[blood sugar|Glycemic]] Targets | |||
|- | |||
| colspan="3" |OR | |||
|- | |||
|Intensive [[Insulin]] [[Therapy]] (IIT) | |||
|Physiologic [[Insulin]]-1:1 basal:bolus ratio SQ | |||
Basal: [[insulin glargine|Glargine]] QD or [[insulin detemir|Detemir]] QD-BID | |||
Bolus: RAI (or Reg) before each meal: If meal skipped, skip dose | |||
. '''Premeal [[insulin]] dose include'''s: | |||
1. [[Insulin]] to cover [[carbohydrate]] ingested; 1 unit RAI covers 500/TDI grams [[carbohydrate]] from meal | |||
2. Additional [[insulin]] to correct for high SMBG; 1 unit RAI lowers PG by approximately 1800/TDI mg/dL. (Reg lowers [[blood sugar|PG]] by ~1500/TDI) | |||
3. Consider adjustment for exercise | |||
== | '''Total Daily [[Insulin]]'''5 : 0.3-0.5 units/kg/day and titrate to [[blood sugar|glycemic]] targets | ||
|Follow [[Glycosylated hemoglobin|A1c]] Every 3-6 months and Adjust Regimen to Maintain [[blood sugar|glycemic]] Targets | |||
|- | |||
| colspan="2" |Pramlintide Consider as adjunct [[therapy]] to [[insulin]] in [[patient|patients]] unable to stabilize PPG. | |||
| | |||
|- | |||
| colspan="3" |'''Footnotes''' | |||
1 Consider referring all [[diabetes mellitus type 1|type 1]] [[patient|patients]] to pediatric/adult endocrinologist/comprehensive diabetes specialty team, and consider continuous [[glucose]] monitoring. If [[insulin pump]] [[therapy]] is considered-refer to Certified Pump Trainer. | |||
2 Modern [[glucose]] meters give values corrected to [[blood sugar|plasma glucose]]. | |||
3 Most [[diabetes mellitus type 1|type 1]] [[patient|patients]] need IIT to attain [[blood sugar|glycemic]] targets; IIT may be by SQ multiple [[Injection (medicine)|injection]] or by SQ continuous [[insulin pump]]. | |||
4 Dosages may differ in children and adolescents. | |||
5 Dosage does not depend on [[patient]]'s race.<ref name="ChalewKamps2020">{{cite journal|last1=Chalew|first1=Stuart|last2=Kamps|first2=Jodi|last3=Jurgen|first3=Brittney|last4=Gomez|first4=Ricardo|last5=Hempe|first5=James|title=The relationship of glycemic control, insulin dose, and race with hypoglycemia in youth with type 1 diabetes|journal=Journal of Diabetes and its Complications|volume=34|issue=6|year=2020|pages=107519|issn=10568727|doi=10.1016/j.jdiacomp.2019.107519}}</ref> | |||
6 Twice daily dosing may be required at low basal [[insulin]] doses. | |||
7 Strongly recommend referral to Registered/Licensed Dietitian or Certified [[Diabetes]] Educator with experience in [[diabetes]] [[diet (nutrition)|nutrition]] counseling. | |||
8 Consider decreasing 1 unit for every 30 minute s of vigorous [[Physical exercise|physical activity]]. | |||
|} | |||
* [[Insulin]] receiving [[patient|patients]] who took Sotagliflozin had better [[blood sugar|glycemic]] control compared to [[Scientific control|control group]] with [[Glycosylated hemoglobin|glycated hemoglobin]] level ([[Glycosylated hemoglobin|HbA1c]]) lower than 7.0% and lower risk of sever [[hypoglycemia]]. Although chance of [[diabetic ketoacidosis]] is higher with Sotagliflozin.<ref name="GargHenry2017">{{cite journal|last1=Garg|first1=Satish K.|last2=Henry|first2=Robert R.|last3=Banks|first3=Phillip|last4=Buse|first4=John B.|last5=Davies|first5=Melanie J.|last6=Fulcher|first6=Gregory R.|last7=Pozzilli|first7=Paolo|last8=Gesty-Palmer|first8=Diane|last9=Lapuerta|first9=Pablo|last10=Simó|first10=Rafael|last11=Danne|first11=Thomas|last12=McGuire|first12=Darren K.|last13=Kushner|first13=Jake A.|last14=Peters|first14=Anne|last15=Strumph|first15=Paul|title=Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes|journal=New England Journal of Medicine|volume=377|issue=24|year=2017|pages=2337–2348|issn=0028-4793|doi=10.1056/NEJMoa1708337}}</ref> | |||
{| class="wikitable" | |||
! colspan="4" |[[blood sugar|Glycemic]] Goals | |||
|- | |||
| colspan="4" |Individualize goal based on [[patient]] [[risk factor|risk factors]] | |||
|- | |||
|[[Glycosylated hemoglobin|HbA1c]] | |||
|≤6% | |||
|≤7% | |||
|≤8% | |||
|- | |||
|[[blood sugar|FPG]] | |||
|≤110 | |||
|120 | |||
|140 mg/dl | |||
|- | |||
|2h PP | |||
|≤130 | |||
|180 | |||
|180 mg/dl | |||
|- | |||
| colspan="4" |Intensify management if: Absent/stable [[cardiovascular disease]], mild-moderate microvascular [[Complication (medicine)|complications]], intact [[hypoglycemia]] awareness, infrequent [[hypoglycemia|hypoglycemic]] episodes, recently diagnosed [[diabetes]]. Less intensive management if: Evidence of advanced or poorly controlled [[cardiovascular disease|cardiovascular]] and/or microvascular [[Complication (medicine)|complications]], [[hypoglycemia]] awareness, vulnerable [[patient]] (ie, impaired cognition, [[dementia]], fall history). See “[[Glycosylated hemoglobin|A1c]] Goal” [[treatment]] strategy for further explanation. [[Glycosylated hemoglobin|A1c]] is referenced to a non-diabetic range of 4-6% using a DCCT-based assay. ADA Clinical Practice Recommendations. [[Diabetes]] Care 2009;32(suppl 1):S19-20. | |||
|} | |||
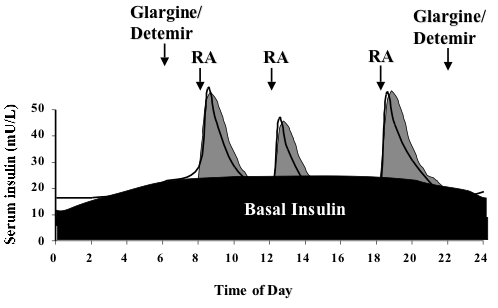
[[File:Insulin basal bolus.png|center|thumb|498x498px|Diagram explaining the basal-bolus insulin schedule. The long acting insulin is given once (usually glargine, Lantus) or twice (usually detemir, Levemir) daily to provide a base, or basal insulin level. Rapid acting (RA) insulin is given before meals and snacks. A similar profile can be provided using an insulin pump where rapid acting insulin is given as the basal and premeal bolus insulin.]] | |||
{| class="wikitable" | |||
! colspan="2" |[[Insulin]] Delivery | |||
|- | |||
!Modes of [[Insulin]] delivery | |||
! | |||
|- | |||
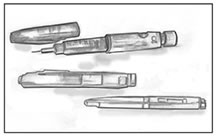
|'''Syringe''' | |||
|'''Syringe''' is a device with a hollow center, plunger, needle, and removable needle guard. | |||
|- | |||
|'''[[Insulin]] pens''' | |||
|'''[[Insulin]] pens''' provide a convenient, easy-to-use way of [[injection (medicine)|injecting]] [[insulin]] and may be less painful than a standard needle and syringe. An [[insulin]] pen looks like a pen with a cartridge. Some of these devices use replaceable cartridges of [[insulin]]. Other pens are prefilled with [[insulin]] and are totally disposable after the [[insulin]] is injected. [[Insulin]] pen users screw a short, fine, disposable needle on the tip of the pen before an [[injection (medicine)|injection]]. Then users turn a dial to select the desired dose of [[insulin]], inject the needle, and press a plunger on the end to deliver the [[insulin]] just under the [[skin]]. [[Insulin]] pens are less widely used in the United States than in many other countries. | |||
|- | |||
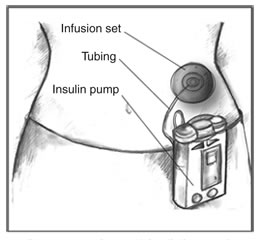
|'''External [[insulin pump]]''' | |||
|'''External [[insulin pumps]]''' are typically about the size of a deck of cards or cell phone, weigh about 3 ounces, and can be worn on a belt or carried in a pocket. Most pumps use a disposable plastic cartridge as an [[insulin]] reservoir. A needle and plunger are temporarily attached to the cartridge to allow the user to fill the cartridge with [[insulin]] from a vial. The user then removes the needle and plunger and loads the filled cartridge into the pump. Disposable [[Intravenous therapy|infusion]] sets are used with [[insulin pump]]s to deliver [[insulin]] to an [[Intravenous therapy|infusion]] site on the body, such as the abdomen. Infusion sets include a cannula—a needle or a small, soft tube—that the user inserts into the [[tissue]] beneath the [[skin]]. Devices are available to help insert the cannula. Narrow, flexible plastic tubing carries [[insulin]] from the pump to the [[Intravenous therapy|infusion]] site. On the [[skin]]'s surface, an adhesive patch or dressing holds the [[Intravenous therapy|infusion]] set in place until the user replaces it after a few days. Users set the pumps to give a steady trickle or "basal" amount of [[insulin]] continuously throughout the day. Pumps can also give "bolus" doses—one-time larger doses—of [[insulin]] at meals and at times when [[blood sugar|blood glucose]] is too high based on the programming set by the user. Frequent [[blood sugar|blood glucose]] monitoring is essential to determine [[insulin]] dosages and to ensure that [[insulin]] is delivered. | |||
|- | |||
|'''[[Injection (medicine)|Injection]] ports''' | |||
|'''[[Injection (medicine)|Injection]] ports''' provide an alternative to daily [[Injection (medicine)|injections]]. [[Injection (medicine)|Injection]] ports look like [[Intravenous therapy|infusion]] sets without the long tubing. Like [[Intravenous therapy|infusion]] sets, [[Injection (medicine)|injections]] ports have a cannula that is inserted into the [[tissue]] beneath the [[skin]]. On the [[skin]]'s surface, an adhesive patch or dressing holds the port in place. The user injects [[insulin]] through the port with a needle and syringe or an [[insulin]] pen. The port remains in place for several days and is then replaced. Use of an [[Injection (medicine)|injection]] port allows a person to reduce the number of [[skin]] punctures to one every few days to apply a new port. | |||
|- | |||
|'''[[Injection (medicine)|Injection]] aids''' | |||
|'''[[Injection (medicine)|Injection]] aids''' are devices that help users give [[Injection (medicine)|injections]] with needles and syringes through the use of spring-loaded syringe holders or stabilizing guides. Many [[Injection (medicine)|injection]] aids have a button the user pushes to inject the [[insulin]]. | |||
|- | |||
|'''[[Insulin]] jet injectors''' | |||
|'''[[Insulin]] jet injectors''' send a fine spray of [[insulin]] into the [[skin]] at high pressure instead of using a needle to deliver the [[insulin]]. | |||
|- | |||
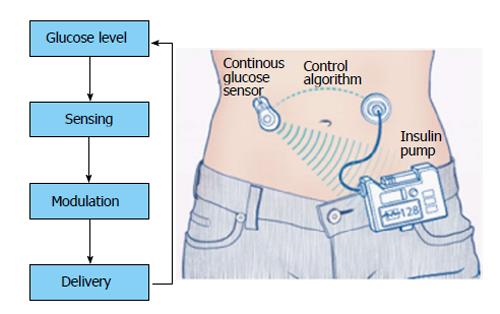
|'''Hybrid Closed-Loop [[Insulin]] Delivery System''' | |||
|A sensor monitors the [[Blood sugar|blood glucose]] constantly and automated algorithmic adjust the basal [[insulin]] [[Intravenous therapy|infusion]] rate. Same as the prior forms, it has a [[Intravenous therapy|infusion]] set which provide [[insulin]]. This method has been linked to few device related [[Adverse effect (medicine)|side effects]].<ref name="BergenstalGarg2016">{{cite journal|last1=Bergenstal|first1=Richard M.|last2=Garg|first2=Satish|last3=Weinzimer|first3=Stuart A.|last4=Buckingham|first4=Bruce A.|last5=Bode|first5=Bruce W.|last6=Tamborlane|first6=William V.|last7=Kaufman|first7=Francine R.|title=Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes|journal=JAMA|volume=316|issue=13|year=2016|pages=1407|issn=0098-7484|doi=10.1001/jama.2016.11708}}</ref> | |||
|} | |||
[[File:Insulin needle.jpg|left|thumb|Most people who take insulin use a needle and syringe to inject insulin just under the skin.]] | |||
[[File:Insulin Port.jpg|center|thumb|Using an injection port reduces the number of skin punctures to one every few days to apply a new port. The user injects insulin through the port.]] | |||
[[File:Insulin Pump.jpg|left|thumb|Insulin pumps contain enough insulin for several days. An infusion set carries insulin from the pump to the body through flexible plastic tubing and a soft tube or needle inserted under the skin.]] | |||
[[File:Insulin Pens.jpg|center|thumb|Insulin pens are a convenient alternative to a needle and syringe for insulin injections]] | |||
= | [[File:Closed-Loop Insulin Delivery System.jpg|alt=Closed-Loop Insulin Delivery System |thumb|Hybrid Closed-Loop Insulin Delivery System is one the Insulin delivery systems. <ref>{{Cite web|url=https://www.wjgnet.com/1948-9358/full/v5/i5/WJD-5-689-g004.htm|title=Principle of closed loop system|last=|first=|date=|website=|archive-url=|archive-date=|dead-url=|access-date=}}</ref>|center|432.986x432.986px]] | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
{{WH}} | |||
{{WS}} | |||
[[Category:Endocrinology]] | [[Category:Endocrinology]] | ||
[[Category:Emergency medicine]] | [[Category:Emergency medicine]] | ||
Latest revision as of 19:56, 20 September 2020
|
Diabetes mellitus type 1 Microchapters |
|
Differentiating Diabetes mellitus type 1 from other Diseases |
|
Diagnosis |
|
Treatment |
|
Cardiovascular Disease and Risk Management |
|
Case Studies |
|
Diabetes mellitus Main page |
|
Patient Information |
|---|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Priyamvada Singh, M.B.B.S. [2]; Cafer Zorkun, M.D., Ph.D. [3]Vishal Devarkonda, M.B.B.S[4]
Overview
See also: Blood glucose monitoring.
The goals of therapy for type 1 or type 2 diabetes mellitus (DM) are to eliminate symptoms related to hyperglycemia, reduce or eliminate the long-term microvascular and macrovascular complications of DM, and allow the patient to achieve as normal lifestyle as possible. Type 1 diabetes is characterized by an absolute insulin deficiency. For these patients, a basal-bolus regimen with a long-acting analog and a short- or rapid-acting insulin analog is the most physiologic insulin regimen and the best option for optimal glycemic control.
Medical Therapy
Type 1 diabetes is characterized by an absolute insulin deficiency. For these patients, a basal-bolus regimen with a long-acting analog and a short- or rapid-acting insulin analog is the most physiologic insulin regimen and the best option for optimal glycemic control. The medical therapy for type 1 DM:[1][2][3]
| Duration of Action of Standard Insulin and Insulin Analogues.* | |||
|---|---|---|---|
| Insulin | Onset of Action | Peak Action | Effective Duration |
| Standard | |||
| Regular | 30-60 min | 2-3 hr | 8-10 hr |
| NPH | 2-4 hr | 4-10 hr | 12-18 hr |
| Zinc insulin(Lente) | 2-4 hr | 4-12 hr | 12-20 hr |
| Extended Zinc insulin | 6-10 hr | 10-16 hr | 18-24 hr |
| Analogues | |||
| Lispro | 5-15 min | 30-90 min | 4-6 hr |
| Aspart | 5-15 min | 30-90 min | 4-6 hr |
| Glargine | 2-4 hr | None | 20-24 hr |
| * Serum insulin profiles are based on a subcutaneous injection of 0.1 to 0.2 unit per kilogram of body weight; large variation within and between persons may be noted. Data are from DeWitt and Hirsch.6 | |||
| Insulin Algorithm for Type 1 Diabetes Mellitus in Children and Adults | ||
|---|---|---|
| ABBREVIATIONS BASAL: Glargine or Detemir BOLUS (Prandial): Reg: Regular Insulin (peak action 3-4 hrs) RAI: Rapid Acting Insulin = Aspart, Glulisine, or Lispro (peak action 1-1 ½ hrs) PPG: Post-Prandial Glucose SMBG: Self-monitored blood glucose TDI: Total daily insulin dosage in units | ||
| Split-Mix Insulin Therapies | 1. Two shots: NPH + Reg or RAI 2:1 ratio AM; 1:1 ratio PM
2. Three shots: AM: NPH + Reg or RAI PM: Reg or RAI HS: NPH 2/3 TDI ÷ as 2/3 AM NPH + 1/3 as Reg or RAI 1/3 TDI ÷ as ½ PM Reg or RAI + ½ NPH at HS 3. Two shots Premix 2/3 AM + 1/3 PM Total Daily Insulin : 0.3-0.5 units/kg/day, and titrate to glycemic targets |
Follow A1c Every 3-6 months and Adjust Regimen to Maintain Glycemic Targets |
| OR | ||
| Intensive Insulin Therapy (IIT) | Physiologic Insulin-1:1 basal:bolus ratio SQ
Basal: Glargine QD or Detemir QD-BID Bolus: RAI (or Reg) before each meal: If meal skipped, skip dose . Premeal insulin dose includes: 1. Insulin to cover carbohydrate ingested; 1 unit RAI covers 500/TDI grams carbohydrate from meal 2. Additional insulin to correct for high SMBG; 1 unit RAI lowers PG by approximately 1800/TDI mg/dL. (Reg lowers PG by ~1500/TDI) 3. Consider adjustment for exercise Total Daily Insulin5 : 0.3-0.5 units/kg/day and titrate to glycemic targets |
Follow A1c Every 3-6 months and Adjust Regimen to Maintain glycemic Targets |
| Pramlintide Consider as adjunct therapy to insulin in patients unable to stabilize PPG. | ||
| Footnotes
1 Consider referring all type 1 patients to pediatric/adult endocrinologist/comprehensive diabetes specialty team, and consider continuous glucose monitoring. If insulin pump therapy is considered-refer to Certified Pump Trainer. 2 Modern glucose meters give values corrected to plasma glucose. 3 Most type 1 patients need IIT to attain glycemic targets; IIT may be by SQ multiple injection or by SQ continuous insulin pump. 4 Dosages may differ in children and adolescents. 5 Dosage does not depend on patient's race.[4] 6 Twice daily dosing may be required at low basal insulin doses. 7 Strongly recommend referral to Registered/Licensed Dietitian or Certified Diabetes Educator with experience in diabetes nutrition counseling. 8 Consider decreasing 1 unit for every 30 minute s of vigorous physical activity. | ||
- Insulin receiving patients who took Sotagliflozin had better glycemic control compared to control group with glycated hemoglobin level (HbA1c) lower than 7.0% and lower risk of sever hypoglycemia. Although chance of diabetic ketoacidosis is higher with Sotagliflozin.[5]
| Glycemic Goals | |||
|---|---|---|---|
| Individualize goal based on patient risk factors | |||
| HbA1c | ≤6% | ≤7% | ≤8% |
| FPG | ≤110 | 120 | 140 mg/dl |
| 2h PP | ≤130 | 180 | 180 mg/dl |
| Intensify management if: Absent/stable cardiovascular disease, mild-moderate microvascular complications, intact hypoglycemia awareness, infrequent hypoglycemic episodes, recently diagnosed diabetes. Less intensive management if: Evidence of advanced or poorly controlled cardiovascular and/or microvascular complications, hypoglycemia awareness, vulnerable patient (ie, impaired cognition, dementia, fall history). See “A1c Goal” treatment strategy for further explanation. A1c is referenced to a non-diabetic range of 4-6% using a DCCT-based assay. ADA Clinical Practice Recommendations. Diabetes Care 2009;32(suppl 1):S19-20. | |||
| Insulin Delivery | |
|---|---|
| Modes of Insulin delivery | |
| Syringe | Syringe is a device with a hollow center, plunger, needle, and removable needle guard. |
| Insulin pens | Insulin pens provide a convenient, easy-to-use way of injecting insulin and may be less painful than a standard needle and syringe. An insulin pen looks like a pen with a cartridge. Some of these devices use replaceable cartridges of insulin. Other pens are prefilled with insulin and are totally disposable after the insulin is injected. Insulin pen users screw a short, fine, disposable needle on the tip of the pen before an injection. Then users turn a dial to select the desired dose of insulin, inject the needle, and press a plunger on the end to deliver the insulin just under the skin. Insulin pens are less widely used in the United States than in many other countries. |
| External insulin pump | External insulin pumps are typically about the size of a deck of cards or cell phone, weigh about 3 ounces, and can be worn on a belt or carried in a pocket. Most pumps use a disposable plastic cartridge as an insulin reservoir. A needle and plunger are temporarily attached to the cartridge to allow the user to fill the cartridge with insulin from a vial. The user then removes the needle and plunger and loads the filled cartridge into the pump. Disposable infusion sets are used with insulin pumps to deliver insulin to an infusion site on the body, such as the abdomen. Infusion sets include a cannula—a needle or a small, soft tube—that the user inserts into the tissue beneath the skin. Devices are available to help insert the cannula. Narrow, flexible plastic tubing carries insulin from the pump to the infusion site. On the skin's surface, an adhesive patch or dressing holds the infusion set in place until the user replaces it after a few days. Users set the pumps to give a steady trickle or "basal" amount of insulin continuously throughout the day. Pumps can also give "bolus" doses—one-time larger doses—of insulin at meals and at times when blood glucose is too high based on the programming set by the user. Frequent blood glucose monitoring is essential to determine insulin dosages and to ensure that insulin is delivered. |
| Injection ports | Injection ports provide an alternative to daily injections. Injection ports look like infusion sets without the long tubing. Like infusion sets, injections ports have a cannula that is inserted into the tissue beneath the skin. On the skin's surface, an adhesive patch or dressing holds the port in place. The user injects insulin through the port with a needle and syringe or an insulin pen. The port remains in place for several days and is then replaced. Use of an injection port allows a person to reduce the number of skin punctures to one every few days to apply a new port. |
| Injection aids | Injection aids are devices that help users give injections with needles and syringes through the use of spring-loaded syringe holders or stabilizing guides. Many injection aids have a button the user pushes to inject the insulin. |
| Insulin jet injectors | Insulin jet injectors send a fine spray of insulin into the skin at high pressure instead of using a needle to deliver the insulin. |
| Hybrid Closed-Loop Insulin Delivery System | A sensor monitors the blood glucose constantly and automated algorithmic adjust the basal insulin infusion rate. Same as the prior forms, it has a infusion set which provide insulin. This method has been linked to few device related side effects.[6] |


References
- ↑ Type 1 Diabetes mellitus "Dennis Kasper, Anthony Fauci, Stephen Hauser, Dan Longo, J. Larry Jameson, Joseph Loscalzo"Harrison's Principles of Internal Medicine, 19e Accessed on December 27th,2016
- ↑ Strich D, Balagour L, Shenker J, Gillis D (2016). "Lower Basal Insulin Dose is Associated with Better Control in Type 1 Diabetes". J Pediatr. doi:10.1016/j.jpeds.2016.11.029. PMID 27974167.
- ↑ "Insulin treatment algorithms" (PDF).
- ↑ Chalew, Stuart; Kamps, Jodi; Jurgen, Brittney; Gomez, Ricardo; Hempe, James (2020). "The relationship of glycemic control, insulin dose, and race with hypoglycemia in youth with type 1 diabetes". Journal of Diabetes and its Complications. 34 (6): 107519. doi:10.1016/j.jdiacomp.2019.107519. ISSN 1056-8727.
- ↑ Garg, Satish K.; Henry, Robert R.; Banks, Phillip; Buse, John B.; Davies, Melanie J.; Fulcher, Gregory R.; Pozzilli, Paolo; Gesty-Palmer, Diane; Lapuerta, Pablo; Simó, Rafael; Danne, Thomas; McGuire, Darren K.; Kushner, Jake A.; Peters, Anne; Strumph, Paul (2017). "Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes". New England Journal of Medicine. 377 (24): 2337–2348. doi:10.1056/NEJMoa1708337. ISSN 0028-4793.
- ↑ Bergenstal, Richard M.; Garg, Satish; Weinzimer, Stuart A.; Buckingham, Bruce A.; Bode, Bruce W.; Tamborlane, William V.; Kaufman, Francine R. (2016). "Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes". JAMA. 316 (13): 1407. doi:10.1001/jama.2016.11708. ISSN 0098-7484.
- ↑ "Principle of closed loop system".