Clindamycin phosphate (gel): Difference between revisions
No edit summary |
m (Protected "Clindamycin phosphate (gel)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (17 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|aOrAn= | |authorTag={{Ammu}} | ||
|genericName=Clindamycin phosphate | |||
|aOrAn=an | |||
|drugClass=[[antibiotic]] | |||
|indicationType=treatment | |indicationType=treatment | ||
| | |indication=[[acne vulgaris]] | ||
|adverseReactions= | |adverseReactions=[[abdominal pain]], [[pseudomembranous colitis]], [[esophagitis]], [[nausea]], [[vomiting]], and [[diarrhea]] | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 12: | Line 15: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult====== | |fdaLIADAdult======Indication===== | ||
* ClindaMax® (Clindamycin Phosphate Gel) is indicated in the treatment of [[acne vulgaris]]. In view of the potential for [[diarrhea]], bloody [[diarrhea]] and [[pseudomembranous colitis]], the physician should consider whether other agents are more appropriate. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=* ClindaMax® (Clindamycin Phosphate Gel) is contraindicated in individuals with a history of [[hypersensitivity]] to preparations containing clindamycin or [[lincomycin]], a history of regional [[enteritis]] or [[ulcerative colitis]], or a history of [[antibiotic-associated colitis]]. | ||
|warnings=* Orally and parenterally administered clindamycin has been associated with severe [[colitis]] which may result in patient death. Use of the topical formulation of clindamycin results in absorption of the [[antibiotic]] from the skin surface. [[Diarrhea]], [[bloody diarrhea]], and [[colitis]] (including [[pseudomembranous colitis]]) have been reported with the use of topical and systemic clindamycin. | |||
* Studies indicate a toxin(s) produced by [[clostridia]] is one primary cause of [[antibiotic-associated colitis]]. The [[colitis]] is usually characterized by severe persistent [[diarrhea]] and severe [[abdominal cramps]] and may be associated with the passage of [[blood]] and [[mucus]]. Endoscopic examination may reveal [[pseudomembranous colitis]]. [[Stool cultures]] for [[Clostridium difficile]] and stool assay for [[C. difficile]] [[toxin]] may be helpful diagnostically. | |||
|warnings=* | * When significant [[diarrhea]] occurs, the drug should be discontinued. [[Colonoscopy|Large bowel endoscopy]] should be considered to establish a definitive diagnosis in cases of severe [[diarrhea]]. | ||
* [[Antiperistaltic agents]] such as [[opiates]] and [[diphenoxylate]] with [[atropine]] may prolong and/or worsen the condition. [[Vancomycin]] has been found to be effective in the treatment of [[antibiotic-associated pseudomembranous colitis]] produced by [[Clostridium difficile]]. The usual adult dosage is 500 mg to 2 grams of [[vancomycin]] orally per day in three to four divided doses administered for 7 to 10 days. [[Cholestyramine]] or [[colestipol]] resins bind [[vancomycin]] in vitro. If both a [[resin]] and [[vancomycin]] are to be administered concurrently, it may be advisable to separate the time of administration of each drug. | |||
* [[Diarrhea]], [[colitis]], and [[pseudomembranous colitis]] have been observed to begin up to several weeks following cessation of oral and parenteral therapy with clindamycin. | |||
|clinicalTrials=* In 18 clinical studies of various formulations of Clindamycin Phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent adverse dermatologic events. | |||
* | : [[File:Clindamycin gel AE 01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Orally and parenterally administered clindamycin has been associated with severe [[colitis]] which may end fatally. | |||
* Cases of [[diarrhea]], [[bloody diarrhea]] and [[colitis]] (including [[pseudomembranous colitis]]) have been reported as adverse reactions in patients treated with oral and parenteral formulations of [[clindamycin]] and rarely with topical clindamycin. | |||
* [[Abdominal pain]] and gastrointestinal disturbances as well as [[gram-negative]] [[folliculitis]] have also been reported in association with the use of topical formulations of clindamycin. | |||
= | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|drugInteractions=* Clindamycin has been shown to have [[neuromuscular blocking agents|neuromuscular blocking]] properties that may enhance the action of other [[neuromuscular blocking agents]] Therefore it should be used with caution in patients receiving such agents. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=* Reproduction studies have been performed in rats and mice using [[subcutaneous]] and oral doses of clindamycin ranging from 100 to 600 mg/kg/day and have revealed no evidence of impaired [[fertility]] or harm to the fetus due to clindamycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
= | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* It is not known whether clindamycin is excreted in human milk following use of clindamycin phosphate. However, orally and parenterally administered clindamycin has been reported to appear in [[breast milk]]. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
|useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
| Line 264: | Line 66: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* [[Topical]] | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 275: | Line 73: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose==== | |overdose=Topically applied clindamycin topical solution can be absorbed in sufficient amounts to produce systemic effects. | ||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 457629722 | |||
| IUPAC_name = methyl 7-chloro-6,7,8-trideoxy-6-{[(4''R'')-1-methyl-4-propyl-<small>L</small>-prolyl]amino}-1-thio-<small>L</small>-threo-α-<small>D</small>-galacto-octopyranoside | |||
| image = Clindamycin skeletal improved.png | |||
| width = 250 | |||
| image2 = Clindamycin 3D 3jz0.png | |||
| width2 = 250 | |||
==== | <!--Clinical data--> | ||
| tradename = Cleocin, Dalacin | |||
| Drugs.com = {{drugs.com|monograph|clindamycin-hydrochloride}} | |||
| MedlinePlus = a682399 | |||
| licence_US = Clindamycin | |||
| pregnancy_AU = A | |||
| pregnancy_US = B | |||
| legal_AU = S4 | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| routes_of_administration = Oral, [[topical]], [[intravenous therapy|IV]], [[pessary|intravaginal]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 90% (oral)<br />4–5% (topical) | |||
| protein_bound = 95% | |||
| metabolism = [[Liver|Hepatic]] | |||
| elimination_half-life = 2–3 hours | |||
| excretion = Biliary and [[kidney|renal]] (around 20%) | |||
==== | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 18323-44-9 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = FF01 | |||
| ATC_supplemental = {{ATC|D10|AF01}} {{ATC|G01|AA10}} | |||
| PubChem = 446598 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB01190 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 393915 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 3745 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 3U02EL437C | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00277 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 2303635 | |||
* | <!--Chemical data--> | ||
| C=18 | H=33 | Cl=1 | N=2 | O=5 | S=1 | |||
| molecular_weight = 424.98 g/mol | |||
| smiles = Cl[C@@H](C)[C@@H](NC(=O)[C@H]1N(C)C[C@H](CCC)C1)[C@H]2O[C@H](SC)[C@H](O)[C@@H](O)[C@H]2O | |||
| InChI = 1/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1 | |||
| InChIKey = KDLRVYVGXIQJDK-AWPVFWJPBP | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H33ClN2O5S/c1-5-6-10-7-11(21(3)8-10)17(25)20-12(9(2)19)16-14(23)13(22)15(24)18(26-16)27-4/h9-16,18,22-24H,5-8H2,1-4H3,(H,20,25)/t9-,10+,11-,12+,13-,14+,15+,16+,18+/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = KDLRVYVGXIQJDK-AWPVFWJPSA-N | |||
| synonyms = 7-chloro-lincomycin<br />7-chloro-7-deoxylincomycin | |||
}} | |||
|mechAction= | |||
* Clindamycin has a primarily [[bacteriostatic]] effect. It is a bacterial [[protein synthesis inhibitor]] by inhibiting ribosomal translocation,<ref>[http://sitemaker.umich.edu/mc3/clindamycin Clindamycin] University of Michigan. Retrieved July 31, 2009</ref> in a similar way to [[macrolide]]s. It does so by binding to the [[50S]] rRNA of the large bacterial [[ribosome]] subunit.<ref name=Merck>{{cite web | url = http://www.merck.com/mmpe/print/sec14/ch170/ch170g.html | title = Lincosamides, Oxazolidinones, and Streptogramins |date=November 2005 | accessdate = 2007-12-01 | publisher = [[Merck & Co.]] | work = [[Merck Manual of Diagnosis and Therapy]]}}</ref> | |||
=== | * The structures of the complexes between several antibiotics (including clindamycin) and a ''[[Deinococcus radiodurans]]'' ribosome have been solved by [[X-ray crystallography]] by a team from the [[Max Planck Society|Max Planck Working Groups for Structural Molecular Biology]], and published in the journal ''[[Nature (journal)|Nature]]''.<ref>{{cite journal |author=Schlünzen F, Zarivach R, Harms J |title=Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria |journal=[[Nature (journal)|Nature]] |volume=413 |issue=6858 |pages=814–21 |year=2001 |pmid=11677599 |doi=10.1038/35101544|display-authors=etal}}</ref> | ||
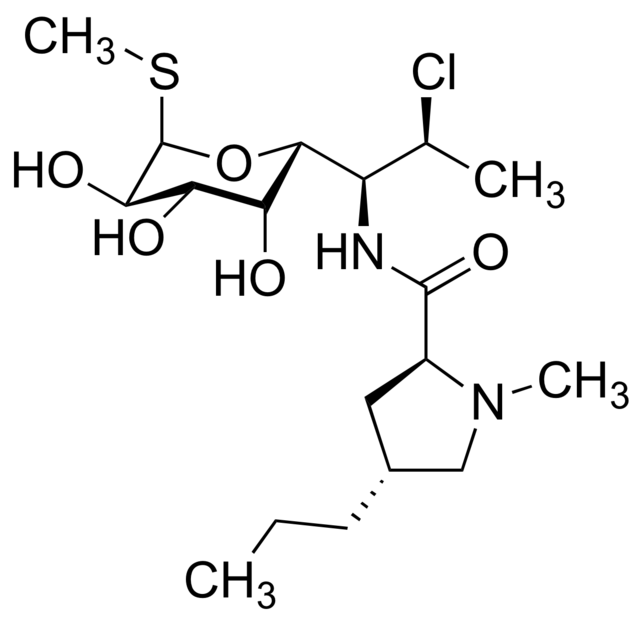
There is limited information regarding <i> | |structure=[[File:clindamycin structure.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!-- | <!--Pharmacokinetics--> | ||
|PK=Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin. | |||
Cross resistance has been demonstrated between clindamycin and lincomycin. | |||
Antagonism has been demonstrated between clindamycin and erythromycin. | |||
Following multiple topical applications of clindamycin phosphate at a concentration equivalent to 10 mg clindamycin per mL in an isopropyl alcohol and water solution, very low levels of clindamycin are present in the serum (0-3 ng/mL) and less than 0.2% of the dose is recovered in urine as clindamycin. | |||
Clindamycin activity has been demonstrated in comedones from acne patients. The mean concentration of [[antibiotic]] activity in extracted comedones after application of Clindamycin Phosphate Topical Solution for 4 weeks was 597 mcg/g of comedonal material (range 0 - 1490). Clindamycin in vitro inhibits all Propionibacterium acnes cultures tested (MICs 0.4 mcg/mL). Free fatty acids on the skin surface have been decreased from approximately 14% to 2% following application of clindamycin. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | ||
| Line 313: | Line 159: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* ClindaMax® Gel (Clindamycin Phosphate Gel USP, 1%) containing clindamycin phosphate equivalent to 10 mg clindamycin per gram is available in the following sizes: | ||
| | * 30 gram tube NDC 0462-0390-30 60 gram tube NDC 0462-0390-60 | ||
|storage=* Store at controlled room temperature 15°-30°C (59°-86°F). Protect from freezing. | |||
|packLabel=[[File:Clinda gel 02.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Clinda gel 03.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Clinda gel 04.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Clinda gel 05.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:DailyMed CLINDAMAX clindamycin phosphate gel.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
| Line 321: | Line 173: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* CLINDAMAX®<ref>{{Cite web | title =CLINDAMAX- clindamycin phosphate gel | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ceee427-50d3-4bfe-8ccf-9efb11a90fb7}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Amides]] | |||
Latest revision as of 19:13, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Clindamycin phosphate (gel) is an antibiotic that is FDA approved for the treatment of acne vulgaris. Common adverse reactions include abdominal pain, pseudomembranous colitis, esophagitis, nausea, vomiting, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- ClindaMax® (Clindamycin Phosphate Gel) is indicated in the treatment of acne vulgaris. In view of the potential for diarrhea, bloody diarrhea and pseudomembranous colitis, the physician should consider whether other agents are more appropriate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clindamycin phosphate (gel) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clindamycin phosphate (gel) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Clindamycin phosphate (gel) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clindamycin phosphate (gel) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clindamycin phosphate (gel) in pediatric patients.
Contraindications
- ClindaMax® (Clindamycin Phosphate Gel) is contraindicated in individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin, a history of regional enteritis or ulcerative colitis, or a history of antibiotic-associated colitis.
Warnings
- Orally and parenterally administered clindamycin has been associated with severe colitis which may result in patient death. Use of the topical formulation of clindamycin results in absorption of the antibiotic from the skin surface. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin.
- Studies indicate a toxin(s) produced by clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Endoscopic examination may reveal pseudomembranous colitis. Stool cultures for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
- When significant diarrhea occurs, the drug should be discontinued. Large bowel endoscopy should be considered to establish a definitive diagnosis in cases of severe diarrhea.
- Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen the condition. Vancomycin has been found to be effective in the treatment of antibiotic-associated pseudomembranous colitis produced by Clostridium difficile. The usual adult dosage is 500 mg to 2 grams of vancomycin orally per day in three to four divided doses administered for 7 to 10 days. Cholestyramine or colestipol resins bind vancomycin in vitro. If both a resin and vancomycin are to be administered concurrently, it may be advisable to separate the time of administration of each drug.
- Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of oral and parenteral therapy with clindamycin.
Adverse Reactions
Clinical Trials Experience
- In 18 clinical studies of various formulations of Clindamycin Phosphate using placebo vehicle and/or active comparator drugs as controls, patients experienced a number of treatment emergent adverse dermatologic events.
- Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally.
- Cases of diarrhea, bloody diarrhea and colitis (including pseudomembranous colitis) have been reported as adverse reactions in patients treated with oral and parenteral formulations of clindamycin and rarely with topical clindamycin.
- Abdominal pain and gastrointestinal disturbances as well as gram-negative folliculitis have also been reported in association with the use of topical formulations of clindamycin.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Clindamycin phosphate (gel) in the drug label.
Drug Interactions
- Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents Therefore it should be used with caution in patients receiving such agents.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and mice using subcutaneous and oral doses of clindamycin ranging from 100 to 600 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to clindamycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clindamycin phosphate (gel) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clindamycin phosphate (gel) during labor and delivery.
Nursing Mothers
- It is not known whether clindamycin is excreted in human milk following use of clindamycin phosphate. However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Clindamycin phosphate (gel) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Clindamycin phosphate (gel) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Clindamycin phosphate (gel) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clindamycin phosphate (gel) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clindamycin phosphate (gel) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clindamycin phosphate (gel) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clindamycin phosphate (gel) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clindamycin phosphate (gel) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Clindamycin phosphate (gel) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Clindamycin phosphate (gel) in the drug label.
Overdosage
Topically applied clindamycin topical solution can be absorbed in sufficient amounts to produce systemic effects.
Pharmacology
Mechanism of Action
- Clindamycin has a primarily bacteriostatic effect. It is a bacterial protein synthesis inhibitor by inhibiting ribosomal translocation,[1] in a similar way to macrolides. It does so by binding to the 50S rRNA of the large bacterial ribosome subunit.[2]
- The structures of the complexes between several antibiotics (including clindamycin) and a Deinococcus radiodurans ribosome have been solved by X-ray crystallography by a team from the Max Planck Working Groups for Structural Molecular Biology, and published in the journal Nature.[3]
Structure

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Clindamycin phosphate (gel) in the drug label.
Pharmacokinetics
Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin.
Cross resistance has been demonstrated between clindamycin and lincomycin.
Antagonism has been demonstrated between clindamycin and erythromycin.
Following multiple topical applications of clindamycin phosphate at a concentration equivalent to 10 mg clindamycin per mL in an isopropyl alcohol and water solution, very low levels of clindamycin are present in the serum (0-3 ng/mL) and less than 0.2% of the dose is recovered in urine as clindamycin.
Clindamycin activity has been demonstrated in comedones from acne patients. The mean concentration of antibiotic activity in extracted comedones after application of Clindamycin Phosphate Topical Solution for 4 weeks was 597 mcg/g of comedonal material (range 0 - 1490). Clindamycin in vitro inhibits all Propionibacterium acnes cultures tested (MICs 0.4 mcg/mL). Free fatty acids on the skin surface have been decreased from approximately 14% to 2% following application of clindamycin.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Clindamycin phosphate (gel) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Clindamycin phosphate (gel) in the drug label.
How Supplied
- ClindaMax® Gel (Clindamycin Phosphate Gel USP, 1%) containing clindamycin phosphate equivalent to 10 mg clindamycin per gram is available in the following sizes:
- 30 gram tube NDC 0462-0390-30 60 gram tube NDC 0462-0390-60
Storage
- Store at controlled room temperature 15°-30°C (59°-86°F). Protect from freezing.
Images
Drug Images
{{#ask: Page Name::Clindamycin phosphate (gel) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel





{{#ask: Label Page::Clindamycin phosphate (gel) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Clindamycin phosphate (gel) in the drug label.
Precautions with Alcohol
- Alcohol-Clindamycin phosphate (gel) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CLINDAMAX®[4]
Look-Alike Drug Names
There is limited information regarding Clindamycin phosphate (gel) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Clindamycin University of Michigan. Retrieved July 31, 2009
- ↑ "Lincosamides, Oxazolidinones, and Streptogramins". Merck Manual of Diagnosis and Therapy. Merck & Co. November 2005. Retrieved 2007-12-01.
- ↑ Schlünzen F, Zarivach R, Harms J; et al. (2001). "Structural basis for the interaction of antibiotics with the peptidyl transferase centre in eubacteria". Nature. 413 (6858): 814–21. doi:10.1038/35101544. PMID 11677599.
- ↑ "CLINDAMAX- clindamycin phosphate gel".