Cimetidine (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cimetidine (oral) is an antacid that is FDA approved for the treatment of duodenal ulcer disease, erosive esophagitis, gastric ulcer, systemic mast cell disease, Zollinger-Ellison syndrome.. Common adverse reactions include gynecomastia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Short-term treatment of active duodenal ulcer
- Dosage: 800 mg at bedtime
- Most patients heal within 4 weeks and there is rarely reason to use cimetidine tablets at full dosage for longer than 6 to 8 weeks.
- Concomitant antacids should be given as needed for relief of pain.
- However, simultaneous administration of cimetidine tablets and antacids is not recommended, since antacids have been reported to interfere with the absorption of cimetidine.
Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of active ulcer
- Patients have been maintained on continued treatment with cimetidine tablets 400 mg at bedtime for periods of up to 5 years.
Short-term treatment of active benign gastric ulcer
- 800 mg at bedtime
- 300 mg 4 times a day with meals and at bedtime
- There is no information concerning usefulness of treatment periods of longer than 8 weeks.
Erosive gastroesophageal reflux (GERD)
- Dosage: 1600 mg daily in divided doses (800 mg twice daily or 400 mg 4 times daily) for 12 weeks
- Erosive esophagitis diagnosed by endoscopy.
- Treatment is indicated for 12 weeks for healing of lesions and control of symptoms
- The use of cimetidine tablets beyond 12 weeks has not been established.
The treatment of pathological hypersecretory conditions (i.e., Zollinger-Ellison Syndrome, systemic mastocytosis, multiple endocrine adenomas)
- Dosage: 300 mg 4 times a day with meals and at bedtime
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cimetidine in adult patients.
Non–Guideline-Supported Use
Clear cell carcinoma of kidney
- Dosage: 600 mg PO 4 times daily [1]
Contrast media adverse reaction
- 300 mg in 100 mL of D5W over 15 minutes rapidly reversed cutaneous and respiratory phenomenon [2]
Maintenance of Gastric ulcer
- Dosage: 400 mg at bedtime [3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Cimetidine (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cimetidine in pediatric patients.
Non–Guideline-Supported Use
Prophylaxis of aspiration pneumonitis
- Dosage: 7.5 mg/kg PO 1 to 3 hours before surgery [4]
Molluscum contagiosum infection
- Dosage: 40 mg/kg PO daily [5]
- ====Stress ulcer====
- Dosage: 15 to 20 mg/kg PO daily administered IV every 6 hours for 7 days in conjunction with cold saline washes, antacids, and blood transfusions [6]
Verruca vulgaris
- Monotherapy: 30 to 40 mg/kg/day [7] [8] [9]
- Combination therapy: 30 mg/kg orally in 3 divided doses with levamisole [10]
Contraindications
Cimetidine tablets are contraindicated for patients known to have hypersensitivity to the product.
Warnings
Rare instances of cardiac arrhythmias and hypotension have been reported following the rapid administration of cimetidine hydrochloride injection by intravenous bolus.
Symptomatic response to treatment with cimetidine does not preclude the presence of a gastric malignancy. There have been rare reports of transient healing of gastric ulcers despite subsequently documented malignancy.
Reversible confusional states have been observed on occasion, predominantly, but not exclusively, in severely ill patients. Advancing age (50 or more years) and preexisting liver and/or renal disease appear to be contributing factors. In some patients these confusional states have been mild and have not required discontinuation of cimetidine. In cases where discontinuation was judged necessary, the condition usually cleared within 3 to 4 days of drug withdrawal.
Adverse Reactions
Clinical Trials Experience
Gastrointestinal
- Diarrhea (usually mild) has been reported in approximately 1 in 100 patients.
CNS=
- Headaches, ranging from mild to severe, have been reported in 3.5% of 924 patients taking 1600 mg/day, 2.1% of 2,225 patients taking 800 mg/day and 2.3% of 1,897 patients taking placebo.
- Dizziness and somnolence (usually mild) have been reported in approximately 1 in 100 patients on either 1600 mg/day or 800 mg/day.
- Reversible confusional states, e.g., mental confusion, agitation, psychosis, depression, anxiety, hallucinations, disorientation, have been reported predominantly, but not exclusively, in severely ill patients. They have usually developed within 2 to 3 days of initiation of treatment with cimetidine and have cleared within 3 to 4 days of discontinuation of the drug.
Endocrine=
- Gynecomastia has been reported in patients treated for one month or longer. In patients being treated for pathological hypersecretory states, this occurred in about 4% of cases while in all others the incidence was 0.3% to 1% in various studies. No evidence of induced endocrine dysfunction was found, and the condition remained unchanged or returned toward normal with continuing treatment with cimetidine.
- Reversible impotence has been reported in patients with pathological hypersecretory disorders, e.g., Zollinger-Ellison Syndrome, receiving cimetidine, particularly in high doses, for at least 12 months (range 12 to 79 months, mean 38 months). However, in large-scale surveillance studies at regular dosage, the incidence has not exceeded that commonly reported in the general population.
Hematologic
- Decreased white blood cell counts in patients treated with cimetidine (approximately 1 per 100,000 patients), including agranulocytosis (approximately 3 per million patients), have been reported, including a few reports of recurrence on rechallenge. Most of these reports were in patients who had serious concomitant illnesses and received drugs and/or treatment known to produce neutropenia.
- Thrombocytopenia (approximately 3 per million patients) and, very rarely, cases of pancytopenia or aplastic anemia have also been reported.
- As with some other H2-receptor antagonists, there have been extremely rare reports of immune hemolytic anemia.
Hepatobiliary
- Dose related increases in serum transaminase have been reported. In most cases they did not progress with continued therapy and returned to normal at the end of therapy. There have been rare reports of cholestatic or mixed cholestatic-hepatocellular effects. These were usually reversible. Because of the predominance of cholestatic features, severe parenchymal injury is considered highly unlikely. However, as in the occasional liver injury with other H2-receptor antagonists, in exceedingly rare circumstances fatal outcomes have been reported.
- There has been reported a single case of biopsy-proven periportal hepatic fibrosis in a patient receiving cimetidine.
- Rare cases of pancreatitis, which cleared on withdrawal of the drug, have been reported.
Hypersensitivity
- Rare cases of fever and allergic reactions including anaphylaxis and hypersensitivity vasculitis, which cleared on withdrawal of the drug, have been reported.
Renal
- Small, possibly dose related increases in plasma creatinine, presumably due to competition for renal tubular secretion, are not uncommon and do not signify deteriorating renal function. Rare cases of interstitial nephritis and urinary retention, which cleared on withdrawal of the drug, have been reported.
Cardiovascular
- Rare cases of bradycardia, tachycardia and AV heart block have been reported with H2-receptor antagonists.
Musculoskeletal
- There have been rare reports of reversible arthralgia and myalgia; exacerbation of joint symptoms in patients with preexisting arthritis has also been reported. Such symptoms have usually been alleviated by a reduction in the dosage of cimetidine.
- Rare cases of polymyositis have been reported, but no causal relationship has been established.
Integumental
- Mild rash
- Very rarely, cases of severe generalized skin reactions including Stevens-Johnson Syndrome, epidermal necrolysis, erythema multiforme, exfoliative dermatitis and generalized exfoliative erythroderma have been reported with H2-receptor antagonists. * Reversible alopecia has been reported very rarely.
Immune Function
- There have been extremely rare reports of strongyloidiasis hyperinfection in immunocompromised patients.
Respiratory
- A large epidemiological study suggested an increased risk of developing pneumonia in current users of histamine-2-receptor antagonists (H2RAs) compared to patients who had stopped H2RA treatment, with an observed adjusted relative risk of 1.63 (95% CI, 1.07 to 2.48). However, a causal relationship between use of H2RAs and pneumonia has not been established.
Postmarketing Experience
There is limited information regarding Cimetidine (oral) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Cimetidine (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Cimetidine (oral) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cimetidine (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cimetidine (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Cimetidine (oral) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Cimetidine (oral) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Cimetidine (oral) in geriatric settings.
Gender
There is no FDA guidance on the use of Cimetidine (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cimetidine (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cimetidine (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cimetidine (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cimetidine (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cimetidine (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Cimetidine (oral) Administration in the drug label.
Monitoring
There is limited information regarding Cimetidine (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Cimetidine (oral) and IV administrations.
Overdosage
There is limited information regarding Cimetidine (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Cimetidine (oral) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Cimetidine (oral) Mechanism of Action in the drug label.
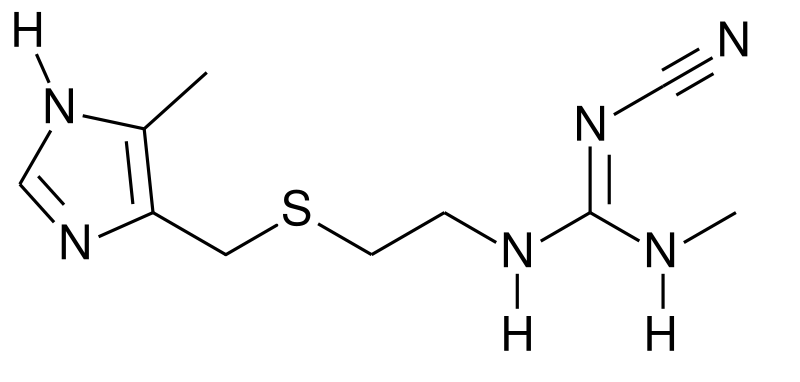
Structure
There is limited information regarding Cimetidine (oral) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Cimetidine (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Cimetidine (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Cimetidine (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Cimetidine (oral) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Cimetidine (oral) How Supplied in the drug label.
Storage
There is limited information regarding Cimetidine (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cimetidine (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cimetidine (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cimetidine (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cimetidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Cimetidine (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Cimetidine (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Inhorn L, Williams SD, Nattam S, Stephens D (1992). "High-dose cimetidine for the treatment of metastatic renal cell carcinoma. A Hoosier Oncology Group study". Am J Clin Oncol. 15 (2): 157–9. PMID 1553905.
- ↑ Myers GE, Bloom FL (1981). "Cimetidine (Tagamet) combined with steroids and H1 antihistamines for the prevention of serious radiographic contrast material reactions". Cathet Cardiovasc Diagn. 7 (1): 65–9. PMID 7214521.
- ↑ Kinloch JD, Pearson AJ, Woolf IL, Young PH (1984). "The effect of cimetidine on the maintenance of healing of gastric ulceration". Postgrad Med J. 60 (708): 665–7. PMC 2418023. PMID 6387689.
- ↑ Goudsouzian N, Coté CJ, Liu LM, Dedrick DF (1981). "The dose-response effects of oral cimetidine on gastric pH and volume in children". Anesthesiology. 55 (5): 533–6. PMID 7294407.
- ↑ Dohil M, Prendiville JS (1996). "Treatment of molluscum contagiosum with oral cimetidine: clinical experience in 13 patients". Pediatr Dermatol. 13 (4): 310–2. PMID 8844752.
- ↑ Agarwal AK, Saili A, Pandey KK, Saxena AK, Sarna MS, Dutta AK (1990). "Role of cimetidine in prevention and treatment of stress induced gastric bleeding in neonates". Indian Pediatr. 27 (5): 465–9. PMID 2276774.
- ↑ Ronna T, Lebwohl M (1995). "Cimetidine therapy for plantar warts". J Am Podiatr Med Assoc. 85 (11): 717–8. doi:10.7547/87507315-85-11-717. PMID 8537909.
- ↑ Choi YS, Hann SK, Park YK (1993). "The effect of cimetidine on verruca plana juvenilis: clinical trials in six patients". J Dermatol. 20 (8): 497–500. PMID 8245312.
- ↑ Orlow SJ, Paller A (1993). "Cimetidine therapy for multiple viral warts in children". J Am Acad Dermatol. 28 (5 Pt 1): 794–6. PMID 8496433.
- ↑ Parsad D, Pandhi R, Juneja A, Negi KS (2001). "Cimetidine and levamisole versus cimetidine alone for recalcitrant warts in children". Pediatr Dermatol. 18 (4): 349–52. PMID 11576414.
 | |
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, parenteral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60–70% |
| Protein binding | 15–20% |
| Metabolism | Hepatic |
| Elimination half-life | 2 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H16N6S |
| Molar mass | 252.34 g/mol |
|
WikiDoc Resources for Cimetidine (oral) |
|
Articles |
|---|
|
Most recent articles on Cimetidine (oral) Most cited articles on Cimetidine (oral) |
|
Media |
|
Powerpoint slides on Cimetidine (oral) |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Cimetidine (oral) |
|
Clinical Trials |
|
Ongoing Trials on Cimetidine (oral) at Clinical Trials.gov Trial results on Cimetidine (oral) Clinical Trials on Cimetidine (oral) at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cimetidine (oral) NICE Guidance on Cimetidine (oral)
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cimetidine (oral) Discussion groups on Cimetidine (oral) Patient Handouts on Cimetidine (oral) Directions to Hospitals Treating Cimetidine (oral) Risk calculators and risk factors for Cimetidine (oral)
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cimetidine (oral) |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Cimetidine (INN) (Template:PronEng) is a histamine H2-receptor antagonist that inhibits the production of acid in the stomach. It is largely used in the treatment of heartburn and peptic ulcers. It is marketed by GlaxoSmithKline under the trade name Tagamet (sometimes Tagamet HB or Tagamet HB200) and was approved by the Food & Drug Administration for prescriptions starting January 1, 1979.
Clinical use
Main article:H2-receptor antagonist
History and development
Cimetidine was the prototypical histamine H2-receptor antagonist from which the later members of the class were developed. Cimetidine was the culmination of a project at Smith, Kline & French (SK&F; now GlaxoSmithKline) to develop a histamine receptor antagonist to suppress stomach acid secretion.
At the time (1964) it was known that histamine was able to stimulate the secretion of stomach acid, but also that traditional antihistamines had no effect on acid production. In the process, the SK&F scientists also proved the existence of histamine H2-receptors.
The SK&F team used a rational drug-design structure starting from the structure of histamine - the only design lead, since nothing was known of the then hypothetical H2-receptor. Hundreds of modified compounds were synthesised in an effort to develop a model of the receptor. The first breakthrough was Nα-guanylhistamine, a partial H2-receptor antagonist. From this lead the receptor model was further refined and eventually led to the development of burimamide, the first H2-receptor antagonist. Burimamide, a specific competitive antagonist at the H2-receptor 100-times more potent than Nα-guanylhistamine, proved the existence of the H2-receptor.
Burimamide was still insufficiently potent for oral administration and further modification of the structure, based on modifying the pKa of the compound, lead to the development of metiamide. Metiamide was an effective agent, however it was associated with unacceptable nephrotoxicity and agranulocytosis. It was proposed that the toxicity arose from the thiourea group, and similar guanidine-analogues were investigated until the ultimate discovery of cimetidine.
Other uses
There have been two studies relating to the use of Cimetidine for treatment of warts in children. According to the studies, a daily dosage of 400mg of Cimetidine can remove over 200 warts from a 15 year old child.[4]
Another study by Yokoyama et al used Cimetidine for the treatment of Chronic Calcifying Tendonitis of the shoulder. The small scale study took 16 individuals with calcifying tendonitis in one shoulder, all of which had previously attempted other forms of therapy including steroid injection and arthroscopic lavage. During the course of the study 10 patients reported an elimination of pain and 9 displayed a complete disappearance of Calcium deposits. With results being on a small scale, it has been recommended that Cimetidine, for the treatment of chronic calcifying tendonitis of the shoulder, be opened to large scale clinical trials. [5]
Cimetidine has also been reported for use in treatment of colorectal cancer - it is however not approved in the US by the FDA for cancer treatment.
Shortcomings and side effects
Cimetidine is a known inhibitor of many isozymes of the cytochrome P450 enzyme system (specifically CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4). This inhibition forms the basis of the numerous drug interactions that occur between cimetidine and other drugs. For example, cimetidine may decrease metabolism of some drugs, such as those used in hormonal contraception. Cimetidine interferes with metabolism of the hormone estrogen, enhancing estrogen activity. In women, this can lead to galactorrhea, whereas in men gynecomastia and a reduced sperm count can result. Adverse drug reactions were also found to be relatively common with Cimetidine, including interactions with the antimalarial medication Hydroxychloroquine.
The development of longer-acting H2-receptor antagonists with reduced adverse effects such as ranitidine proved to be the downfall of cimetidine and, whilst it is still used, it is no longer amongst the more widely used H2-receptor antagonists. Cimetidine should be used with caution is causes of hepatic impairment and cardiovascular disease. Side effects can include dizziness, and more rarely, headache. BIOAVAILABILITY: The observed bioavailibility of cimetidine is almost 60%.
References
- Michnovicz JJ, Galbraith RA .Cimetidine inhibits catechol estrogen metabolism in women. Metabolism. 1991 Feb;40(2):170-4. PMID 1988774
Template:H2-receptor antagonist
de:Cimetidin hr:Cimeditin nl:Cimetidine fi:Simetidiini th:ไซเมติดีน
- CS1 maint: Multiple names: authors list
- CS1 maint: PMC format
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Guanidines
- H2 receptor antagonists
- Imidazoles
- Thioethers
- Drugs