Cilostazol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CONTRAINDICATION
See full prescribing information for complete Boxed Warning.
* Cilostazol and several of its metabolites are inhibitors of phosphodiesterase III. Several drugs with this pharmacologic effect have caused decreased survival compared to placebo in patients with class III-IV congestive heart failure. Cilostazol is contraindicated in patients with congestive heart failure of any severity.
|
Overview
Cilostazol is a phosphodiesterase 3 (PDE3) inhibitor and platelet aggregation inhibitor that is FDA approved for the {{{indicationType}}} of intermittent claudication, as indicated by an increased walking distance. There is a Black Box Warning for this drug as shown here. Common adverse reactions include palpitations, peripheral edema, tachyarrhythmia, abdominal pain, diarrhea, indigestion, decreased platelet aggregation, backache, myalgia, dizziness, headache, cough, pharyngitis, and rhinitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Intermittent Claudication
- Dosing Information
- 100 mg PO bid, taken at least half an hour before or two hours after breakfast and dinner.
- A dose of 50 mg PO bid should be considered during coadministration of such inhibitors of CYP3A4 as ketoconazole, itraconazole, erythromycin and diltiazem, and during coadministration of such inhibitors of CYP2C19 as omeprazole.
- Patients may respond as early as 2 to 4 weeks after the initiation of therapy, but treatment for up to 12 weeks may be needed before a beneficial effect is experienced.
- Discontinuation of Therapy
- The available data suggest that the dosage of cilostazol can be reduced or discontinued without rebound (i.e., platelet hyperaggregability).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Secondary Prophylaxis of Noncardioembolic Ischemic Stroke or TIA
- Developed by: American College of Chest Physicians (ACCP)
- Dosing Information
- In patients with a history of noncardioembolic ischemic stroke or TIA, ACCP recommends long-term treatment with aspirin (75-100 mg once daily), clopidogrel (75 mg once daily), aspirin/extended-release dipyridamole (25 mg/200 mg bid), or cilostazol (100 mg bid) over no antiplatelet therapy (Grade 1A), oral anticoagulants (Grade 1B), the combination of clopidogrel plus aspirin (Grade 1B), or triflusal (Grade 2B).
- Of the recommended antiplatelet regimens, ACCP suggests clopidogrel or aspirin/extended-release dipyridamole over aspirin (Grade 2B) or cilostazol (Grade 2C).
Elective Percutaneous Coronary Intervention with Bare-Metal or Drug-Eluting Stent
- Developed by: American College of Chest Physicians (ACCP)
- Dosing Information
- Use of low-dose aspirin 75 to 100 mg daily and clopidogrel 75 mg daily alone is recommended, rather than cilostazol in addition to these drugs (Grade 1B).
- Aspirin 75 to 100 mg daily and clopidogrel 75 mg daily as part of dual antiplatelet therapy is suggested, rather than the use of either drug with cilostazol (Grade 1B).
- Cilostazol 100 mg twice daily as substitute for either low-dose aspirin 75 to 100 mg daily or clopidogrel 75 mg daily as part of a dual antiplatelet regimen is suggested in patients with an allergy or intolerance of either drug class (Grade 2C).
Non–Guideline-Supported Use
Primary Prophylaxis of Cerebrovascular Accident
- Dosing Information
Prophylaxis of Femoral Artery Occlusion After Percutaneous Coronary Intervention
- Dosing Information
- Cilostazol 200 mg/day plus aspirin 100 mg/day significantly reduced the rate of restenosis of femoropopliteal lesions, and significantly improved event-free survival at 12 months after percutaneous transluminal angioplasty with provisional nitinol stenting when compared with use of aspirin alone.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Cilostazol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Cilostazol in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cilostazol in pediatric patients.
Contraindications
- Condition1
Warnings
|
CONTRAINDICATION
See full prescribing information for complete Boxed Warning.
* Cilostazol and several of its metabolites are inhibitors of phosphodiesterase III. Several drugs with this pharmacologic effect have caused decreased survival compared to placebo in patients with class III-IV congestive heart failure. Cilostazol is contraindicated in patients with congestive heart failure of any severity.
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Cilostazol in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Cilostazol in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cilostazol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cilostazol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Cilostazol with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Cilostazol with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Cilostazol with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Cilostazol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cilostazol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cilostazol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cilostazol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cilostazol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cilostazol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Cilostazol in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Cilostazol in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Cilostazol in the drug label.
Pharmacology
| |
Cilostazol
| |
| Systematic (IUPAC) name | |
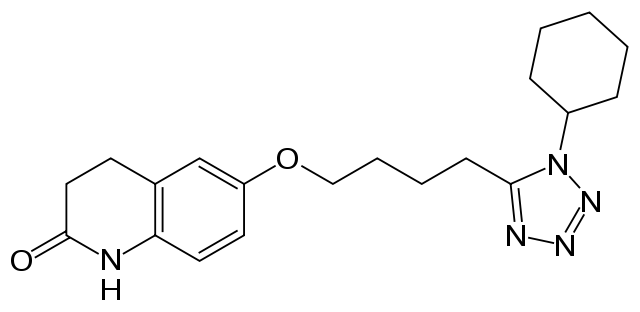
| 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]- 3,4-dihydro-2(1H)-quinolinone | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 369.46 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 95–98% |
| Metabolism | Hepatic (CYP3A4- and CYP2C19-mediated) |
| Half life | 11–13 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cilostazol in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Cilostazol in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Cilostazol in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Cilostazol in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Cilostazol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cilostazol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cilostazol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Cilostazol in the drug label.
Precautions with Alcohol
- Alcohol-Cilostazol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Pletal®[3]
Look-Alike Drug Names
- N/A[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Shinoda-Tagawa, T. (2002-02). "A phosphodiesterase inhibitor, cilostazol, prevents the onset of silent brain infarction in Japanese subjects with Type II diabetes". Diabetologia. 45 (2): 188–194. doi:10.1007/s00125-001-0740-2. ISSN 0012-186X. PMID 11935149. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Ahn, C. W. (2001-04). "Decrease in carotid intima media thickness after 1 year of cilostazol treatment in patients with type 2 diabetes mellitus". Diabetes Research and Clinical Practice. 52 (1): 45–53. ISSN 0168-8227. PMID 11182215. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "CILOSTAZOL tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Cilostazol |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Cilostazol |Label Name=Cilostazol06.png
}}
{{#subobject:
|Label Page=Cilostazol |Label Name=Cilostazol07.png
}}
{{#subobject:
|Label Page=Cilostazol |Label Name=Cilostazol08.png
}}
