Cilostazol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
<!--Overview--> | <!--Overview--> | ||
|genericName= | |genericName= | ||
Cilostazol | Cilostazol | ||
|aOrAn= | |aOrAn= | ||
a | a | ||
|drugClass= | |drugClass= | ||
[[phosphodiesterase inhibitor|phosphodiesterase 3 (PDE3) inhibitor]] and [[platelet aggregation]] [[inhibitor]] | [[phosphodiesterase inhibitor|phosphodiesterase 3 (PDE3) inhibitor]] and [[platelet aggregation]] [[inhibitor]] | ||
|indication= | |indication= | ||
intermittent [[claudication]], as indicated by an increased walking distance | intermittent [[claudication]], as indicated by an increased walking distance | ||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
Yes | Yes | ||
|adverseReactions= | |adverseReactions= | ||
| Line 31: | Line 25: | ||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle= | |blackBoxWarningTitle= | ||
CONTRAINDICATION | CONTRAINDICATION | ||
|blackBoxWarningBody= | |blackBoxWarningBody= | ||
| Line 42: | Line 34: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
| Line 53: | Line 44: | ||
:* Patients may respond as early as 2 to 4 weeks after the initiation of therapy, but treatment for up to 12 weeks may be needed before a beneficial effect is experienced. | :* Patients may respond as early as 2 to 4 weeks after the initiation of therapy, but treatment for up to 12 weeks may be needed before a beneficial effect is experienced. | ||
:* Discontinuation of Therapy | :* Discontinuation of Therapy | ||
::* The available data suggest that the dosage of | ::* The available data suggest that the dosage of Cilostazol can be reduced or discontinued without rebound (i.e., [[platelet]] hyperaggregability). | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport= | ||
| Line 67: | Line 57: | ||
* Dosing Information | * Dosing Information | ||
:* In patients with a history of noncardioembolic ischemic [[stroke]] or [[TIA]], ACCP recommends long-term treatment with [[aspirin]] (75-100 mg once daily), [[clopidogrel]] (75 mg once daily), [[aspirin]]/extended-release [[dipyridamole]] (25 mg/200 mg bid), or ''' | :* In patients with a history of noncardioembolic ischemic [[stroke]] or [[TIA]], ACCP recommends long-term treatment with [[aspirin]] (75-100 mg once daily), [[clopidogrel]] (75 mg once daily), [[aspirin]]/extended-release [[dipyridamole]] (25 mg/200 mg bid), or '''Cilostazol (100 mg bid)''' over no [[antiplatelet]] therapy (Grade 1A), oral [[anticoagulant]]s (Grade 1B), the combination of [[clopidogrel]] plus [[aspirin]] (Grade 1B), or triflusal (Grade 2B). | ||
:* Of the recommended [[antiplatelet]] regimens, ACCP suggests [[clopidogrel]] or [[aspirin]]/extended-release [[dipyridamole]] over [[aspirin]] (Grade 2B) or | :* Of the recommended [[antiplatelet]] regimens, ACCP suggests [[clopidogrel]] or [[aspirin]]/extended-release [[dipyridamole]] over [[aspirin]] (Grade 2B) or Cilostazol (Grade 2C). | ||
=====Elective Percutaneous Coronary Intervention with Bare-Metal or Drug-Eluting Stent===== | =====Elective Percutaneous Coronary Intervention with Bare-Metal or Drug-Eluting Stent===== | ||
| Line 77: | Line 67: | ||
* Dosing Information | * Dosing Information | ||
:* Use of low-dose [[aspirin]] 75 to 100 mg daily and [[clopidogrel]] 75 mg daily alone is recommended, rather than | :* Use of low-dose [[aspirin]] 75 to 100 mg daily and [[clopidogrel]] 75 mg daily alone is recommended, rather than Cilostazol in addition to these drugs (Grade 1B). | ||
:* [[Aspirin]] 75 to 100 mg daily and [[clopidogrel]] 75 mg daily as part of dual [[antiplatelet]] therapy is suggested, rather than the use of either drug with | :* [[Aspirin]] 75 to 100 mg daily and [[clopidogrel]] 75 mg daily as part of dual [[antiplatelet]] therapy is suggested, rather than the use of either drug with Cilostazol (Grade 1B). | ||
:* '''Cilostazol 100 mg twice daily''' as substitute for either low-dose [[aspirin]] 75 to 100 mg daily or [[clopidogrel]] 75 mg daily as part of a dual [[antiplatelet]] regimen is suggested in patients with an [[allergy]] or intolerance of either drug class (Grade 2C). | :* '''Cilostazol 100 mg twice daily''' as substitute for either low-dose [[aspirin]] 75 to 100 mg daily or [[clopidogrel]] 75 mg daily as part of a dual [[antiplatelet]] regimen is suggested in patients with an [[allergy]] or intolerance of either drug class (Grade 2C). | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport= | ||
| Line 89: | Line 78: | ||
* Dosing Information | * Dosing Information | ||
:* '''100–200 mg PO qd'''<ref>{{Cite journal | doi = 10.1007/s00125-001-0740-2 | issn = 0012-186X | volume = 45 | issue = 2 | pages = 188–194 | last = Shinoda-Tagawa | first = T. | coauthors = Y. Yamasaki, S. Yoshida, Y. Kajimoto, T. Tsujino, N. Hakui, M. Matsumoto, M. Hori | title = A phosphodiesterase inhibitor, | :* '''100–200 mg PO qd'''<ref>{{Cite journal | doi = 10.1007/s00125-001-0740-2 | issn = 0012-186X | volume = 45 | issue = 2 | pages = 188–194 | last = Shinoda-Tagawa | first = T. | coauthors = Y. Yamasaki, S. Yoshida, Y. Kajimoto, T. Tsujino, N. Hakui, M. Matsumoto, M. Hori | title = A phosphodiesterase inhibitor, Cilostazol, prevents the onset of silent brain infarction in Japanese subjects with Type II diabetes | journal = Diabetologia | date = 2002-02 | pmid = 11935149 }}</ref><ref>{{Cite journal | issn = 0168-8227 | volume = 52 | issue = 1 | pages = 45–53 | last = Ahn | first = C. W. | coauthors = H. C. Lee, S. W. Park, Y. D. Song, K. B. Huh, S. J. Oh, Y. S. Kim, Y. K. Choi, J. M. Kim, T. H. Lee | title = Decrease in carotid intima media thickness after 1 year of Cilostazol treatment in patients with type 2 diabetes mellitus | journal = Diabetes Research and Clinical Practice | date = 2001-04 | pmid = 11182215 }}</ref> | ||
=====Prophylaxis of Femoral Artery Occlusion After Percutaneous Coronary Intervention===== | =====Prophylaxis of Femoral Artery Occlusion After Percutaneous Coronary Intervention===== | ||
| Line 100: | Line 89: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed= | ||
| Line 108: | Line 96: | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport= | ||
| Line 114: | Line 101: | ||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport= | ||
| Line 120: | Line 106: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications= | ||
| Line 128: | Line 113: | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings= | ||
| Line 134: | Line 118: | ||
* Hematologic Adverse Reactions | * Hematologic Adverse Reactions | ||
:* Rare cases have been reported of [[thrombocytopenia]] or [[leukopenia]] progressing to [[agranulocytosis]] when | :* Rare cases have been reported of [[thrombocytopenia]] or [[leukopenia]] progressing to [[agranulocytosis]] when Cilostazol was not immediately discontinued. The [[agranulocytosis]], however, was reversible on discontinuation of Cilostazol. | ||
* Use with Clopidogrel | * Use with Clopidogrel | ||
:* There is limited information with respect to the efficacy or safety of the concurrent use of | :* There is limited information with respect to the efficacy or safety of the concurrent use of Cilostazol and [[clopidogrel]], a [[platelet]]-aggregation inhibiting drug indicated for use in patients with [[peripheral arterial disease]]. Although it cannot be determined whether there was an additive effect on bleeding times during concomitant administration with Cilostazol and [[clopidogrel]], caution is advised for checking bleeding times during coadministration. | ||
* Hepatic Impairment | * Hepatic Impairment | ||
:* Patients with moderate or severe hepatic impairment have not been studied in clinical trials. Special caution is advised when | :* Patients with moderate or severe hepatic impairment have not been studied in clinical trials. Special caution is advised when Cilostazol is used in such patients. | ||
* Renal Impairment | * Renal Impairment | ||
:* Patients on dialysis have not been studied, but, it is unlikely that | :* Patients on dialysis have not been studied, but, it is unlikely that Cilostazol can be removed efficiently by dialysis because of its high protein binding (95-98%). | ||
:* Special caution is advised when | :* Special caution is advised when Cilostazol is used in patients with severe [[renal impairment]]: estimated [[creatinine clearance]] < 25 mL/min. | ||
* Use with other [[antiplatelet]] agents | * Use with other [[antiplatelet]] agents | ||
:* Cilostazol inhibits [[platelet aggregation]] but in a reversible manner. Caution is advised in patients at risk of bleeding from [[surgery]] or pathologic processes. [[Platelet]] aggregability returns to normal within 96 hours of stopping | :* Cilostazol inhibits [[platelet aggregation]] but in a reversible manner. Caution is advised in patients at risk of bleeding from [[surgery]] or pathologic processes. [[Platelet]] aggregability returns to normal within 96 hours of stopping Cilostazol. Caution is advised in patients receiving both Cilostazol and any other [[antiplatelet]] agent, or in patients with [[thrombocytopenia]]. | ||
* Cardiovascular Toxicity | * Cardiovascular Toxicity | ||
:* Repeated oral administration of | :* Repeated oral administration of Cilostazol to dogs (30 or more mg/kg/day for 52 weeks, 150 or more mg/kg/day for 13 weeks, and 450 mg/kg/day for 2 weeks), produced cardiovascular lesions that included endocardial hemorrhage, [[hemosiderin]] deposition and fibrosis in the left ventricle, [[hemorrhage]] in the right atrial wall, [[hemorrhage]] and necrosis of the smooth muscle in the wall of the coronary artery, intimal thickening of the coronary artery, and coronary arteritis and periarteritis. At the lowest dose associated with cardiovascular lesions in the 52-week study, systemic exposure (AUC) to unbound Cilostazol was less than that seen in humans at the maximum recommended human dose (MRHD) of 100 mg b.i.d. Similar lesions have been reported in dogs following the administration of other positive [[inotropic]] agents (including [[PDE]] III inhibitors) and/or vasodilating agents. No cardiovascular lesions were seen in rats following 5 or 13 weeks of administration of Cilostazol at doses up to 1500 mg/kg/day. At this dose, systemic exposures (AUCs) to unbound Cilostazol were only about 1.5 and 5 times (male and female rats, respectively) the exposure seen in humans at the MRHD. Cardiovascular lesions were also not seen in rats following 52 weeks of administration of Cilostazol at doses up to 150 mg/kg/day. At this dose, systemic exposures (AUCs) to unbound Cilostazol were about 0.5 and 5 times (male and female rats, respectively) the exposure in humans at the MRHD. In female rats, Cilostazol AUCs were similar at 150 and 1500 mg/kg/day. Cardiovascular lesions were also not observed in monkeys after oral administration of Cilostazol for 13 weeks at doses up to 1800 mg/kg/day. While this dose of Cilostazol produced pharmacologic effects in monkeys, plasma Cilostazol levels were less than those seen in humans given the MRHD, and those seen in dogs given doses associated with cardiovascular lesions. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials= | |clinicalTrials= | ||
* Adverse events were assessed in eight placebo-controlled clinical trials involving 2274 patients exposed to either 50 or 100 mg b.i.d. | * Adverse events were assessed in eight placebo-controlled clinical trials involving 2274 patients exposed to either 50 or 100 mg b.i.d. Cilostazol (n=1301) or placebo (n=973), with a median treatment duration of 127 days for patients on Cilostazol and 134 days for patients on placebo. | ||
* The only adverse event resulting in discontinuation of therapy in ≥ 3% of patients treated with | * The only adverse event resulting in discontinuation of therapy in ≥ 3% of patients treated with Cilostazol 50 or 100 mg b.i.d. was headache, which occurred with an incidence of 1.3%, 3.5%, and 0.3% in patients treated with Cilostazol 50 mg b.i.d., 100 mg b.i.d, or placebo, respectively. Other frequent causes of discontinuation included palpitation and diarrhea, both 1.1% for Cilostazol (all doses) versus 0.1% for placebo. | ||
* The most commonly reported adverse events, occurring in ≥ 2% of patients treated with | * The most commonly reported adverse events, occurring in ≥ 2% of patients treated with Cilostazol 50 or 100 mg b.i.d., are shown in the table (below). | ||
: [[File:Cilostazol04.png|600px|thumb|none|This image of the FDA label is provided by the National Library of Medicine.]] | |||
* Other events seen with an incidence of ≥ 2%, but occurring in the placebo group at least as frequently as in the 100 mg b.i.d. group were: [[asthenia]], [[hypertension]], [[vomiting]], leg [[cramps]], [[hypesthesia]], [[paresthesia]], [[dyspnea]], [[rash]], [[hematuria]], [[urinary tract infection]], [[flu]] syndrome, [[angina pectoris]], [[arthritis]], and [[bronchitis]]. | |||
* Less frequent adverse events (<2%) that were experienced by patients exposed to Cilostazol 50 mg b.i.d. or 100 mg b.i.d. in the eight controlled clinical trials and that occurred at a frequency in the 100 mg b.i.d. group greater than in the placebo group, regardless of suspected drug relationship, are listed below. | |||
=====Body as a Whole===== | =====Body as a Whole===== | ||
| Line 218: | Line 199: | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing= | |postmarketing= | ||
| Line 224: | Line 204: | ||
======Central Nervous System====== | ======Central Nervous System====== | ||
======Cardiovascular====== | ======Cardiovascular====== | ||
======Respiratory====== | ======Respiratory====== | ||
======Gastrointestinal====== | ======Gastrointestinal====== | ||
======Hypersensitivity====== | ======Hypersensitivity====== | ||
======Miscellaneous====== | ======Miscellaneous====== | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions= | ||
| Line 255: | Line 222: | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category''' | * '''Pregnancy Category''' | ||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | * '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery= | |useInLaborDelivery= | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | ||
|useInPed= | |useInPed= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | ||
|useInGeri= | |useInGeri= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender= | |useInGender= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace= | |useInRace= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair= | |useInRenalImpair= | ||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair= | |useInHepaticImpair= | ||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential= | |useInReproPotential= | ||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp= | |useInImmunocomp= | ||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration= | |administration= | ||
| Line 301: | Line 255: | ||
* Intravenous | * Intravenous | ||
|monitoring= | |monitoring= | ||
| Line 311: | Line 264: | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat= | |IVCompat= | ||
| Line 317: | Line 269: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose= | ||
| Line 337: | Line 288: | ||
<!--Drugbox2--> | <!--Drugbox2--> | ||
|drugBox= | |drugBox= | ||
| Line 348: | Line 298: | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = Pletal | | tradename = Pletal | ||
| Drugs.com = {{drugs.com|monograph| | | Drugs.com = {{drugs.com|monograph|Cilostazol}} | ||
| MedlinePlus = a601038 | | MedlinePlus = a601038 | ||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| Line 399: | Line 349: | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction= | |mechAction= | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure= | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD= | |PD= | ||
| Line 419: | Line 362: | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK= | ||
| Line 425: | Line 367: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic= | |nonClinToxic= | ||
| Line 431: | Line 372: | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies= | |clinicalStudies= | ||
| Line 441: | Line 381: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied= | |howSupplied= | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo= | |fdaPatientInfo= | ||
| Line 453: | Line 389: | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol= | |alcohol= | ||
| Line 459: | Line 394: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames= | |brandNames= | ||
* Pletal®<ref>{{Cite web | title = | * Pletal®<ref>{{Cite web | title = Cilostazol tablet | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=c4597231-fec0-435d-a0c6-8c237e3d60a9 }}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike= | |lookAlike= | ||
| Line 471: | Line 404: | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
Revision as of 21:32, 8 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CONTRAINDICATION
See full prescribing information for complete Boxed Warning.
* Cilostazol and several of its metabolites are inhibitors of phosphodiesterase III. Several drugs with this pharmacologic effect have caused decreased survival compared to placebo in patients with class III-IV congestive heart failure. Cilostazol is contraindicated in patients with congestive heart failure of any severity.
|
Overview
Cilostazol is a phosphodiesterase 3 (PDE3) inhibitor and platelet aggregation inhibitor that is FDA approved for the {{{indicationType}}} of intermittent claudication, as indicated by an increased walking distance. There is a Black Box Warning for this drug as shown here. Common adverse reactions include palpitations, peripheral edema, tachyarrhythmia, abdominal pain, diarrhea, indigestion, decreased platelet aggregation, backache, myalgia, dizziness, headache, cough, pharyngitis, and rhinitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Intermittent Claudication
- Dosing Information
- 100 mg PO bid, taken at least half an hour before or two hours after breakfast and dinner.
- A dose of 50 mg PO bid should be considered during coadministration of such inhibitors of CYP3A4 as ketoconazole, itraconazole, erythromycin and diltiazem, and during coadministration of such inhibitors of CYP2C19 as omeprazole.
- Patients may respond as early as 2 to 4 weeks after the initiation of therapy, but treatment for up to 12 weeks may be needed before a beneficial effect is experienced.
- Discontinuation of Therapy
- The available data suggest that the dosage of Cilostazol can be reduced or discontinued without rebound (i.e., platelet hyperaggregability).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Secondary Prophylaxis of Noncardioembolic Ischemic Stroke or TIA
- Developed by: American College of Chest Physicians (ACCP)
- Dosing Information
- In patients with a history of noncardioembolic ischemic stroke or TIA, ACCP recommends long-term treatment with aspirin (75-100 mg once daily), clopidogrel (75 mg once daily), aspirin/extended-release dipyridamole (25 mg/200 mg bid), or Cilostazol (100 mg bid) over no antiplatelet therapy (Grade 1A), oral anticoagulants (Grade 1B), the combination of clopidogrel plus aspirin (Grade 1B), or triflusal (Grade 2B).
- Of the recommended antiplatelet regimens, ACCP suggests clopidogrel or aspirin/extended-release dipyridamole over aspirin (Grade 2B) or Cilostazol (Grade 2C).
Elective Percutaneous Coronary Intervention with Bare-Metal or Drug-Eluting Stent
- Developed by: American College of Chest Physicians (ACCP)
- Dosing Information
- Use of low-dose aspirin 75 to 100 mg daily and clopidogrel 75 mg daily alone is recommended, rather than Cilostazol in addition to these drugs (Grade 1B).
- Aspirin 75 to 100 mg daily and clopidogrel 75 mg daily as part of dual antiplatelet therapy is suggested, rather than the use of either drug with Cilostazol (Grade 1B).
- Cilostazol 100 mg twice daily as substitute for either low-dose aspirin 75 to 100 mg daily or clopidogrel 75 mg daily as part of a dual antiplatelet regimen is suggested in patients with an allergy or intolerance of either drug class (Grade 2C).
Non–Guideline-Supported Use
Primary Prophylaxis of Cerebrovascular Accident
- Dosing Information
Prophylaxis of Femoral Artery Occlusion After Percutaneous Coronary Intervention
- Dosing Information
- Cilostazol 200 mg/day plus aspirin 100 mg/day significantly reduced the rate of restenosis of femoropopliteal lesions, and significantly improved event-free survival at 12 months after percutaneous transluminal angioplasty with provisional nitinol stenting when compared with use of aspirin alone.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of Cilostazole in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cilostazol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cilostazol in pediatric patients.
Contraindications
- Congestive heart failure of any severity
- Hemostatic disorders or active pathologic bleeding, such as bleeding peptic ulcer and intracranial bleeding
- Hypersensitivity to any of its components
Warnings
|
CONTRAINDICATION
See full prescribing information for complete Boxed Warning.
* Cilostazol and several of its metabolites are inhibitors of phosphodiesterase III. Several drugs with this pharmacologic effect have caused decreased survival compared to placebo in patients with class III-IV congestive heart failure. Cilostazol is contraindicated in patients with congestive heart failure of any severity.
|
Precautions
- Hematologic Adverse Reactions
- Rare cases have been reported of thrombocytopenia or leukopenia progressing to agranulocytosis when Cilostazol was not immediately discontinued. The agranulocytosis, however, was reversible on discontinuation of Cilostazol.
- Use with Clopidogrel
- There is limited information with respect to the efficacy or safety of the concurrent use of Cilostazol and clopidogrel, a platelet-aggregation inhibiting drug indicated for use in patients with peripheral arterial disease. Although it cannot be determined whether there was an additive effect on bleeding times during concomitant administration with Cilostazol and clopidogrel, caution is advised for checking bleeding times during coadministration.
- Hepatic Impairment
- Patients with moderate or severe hepatic impairment have not been studied in clinical trials. Special caution is advised when Cilostazol is used in such patients.
- Renal Impairment
- Patients on dialysis have not been studied, but, it is unlikely that Cilostazol can be removed efficiently by dialysis because of its high protein binding (95-98%).
- Special caution is advised when Cilostazol is used in patients with severe renal impairment: estimated creatinine clearance < 25 mL/min.
- Use with other antiplatelet agents
- Cilostazol inhibits platelet aggregation but in a reversible manner. Caution is advised in patients at risk of bleeding from surgery or pathologic processes. Platelet aggregability returns to normal within 96 hours of stopping Cilostazol. Caution is advised in patients receiving both Cilostazol and any other antiplatelet agent, or in patients with thrombocytopenia.
- Cardiovascular Toxicity
- Repeated oral administration of Cilostazol to dogs (30 or more mg/kg/day for 52 weeks, 150 or more mg/kg/day for 13 weeks, and 450 mg/kg/day for 2 weeks), produced cardiovascular lesions that included endocardial hemorrhage, hemosiderin deposition and fibrosis in the left ventricle, hemorrhage in the right atrial wall, hemorrhage and necrosis of the smooth muscle in the wall of the coronary artery, intimal thickening of the coronary artery, and coronary arteritis and periarteritis. At the lowest dose associated with cardiovascular lesions in the 52-week study, systemic exposure (AUC) to unbound Cilostazol was less than that seen in humans at the maximum recommended human dose (MRHD) of 100 mg b.i.d. Similar lesions have been reported in dogs following the administration of other positive inotropic agents (including PDE III inhibitors) and/or vasodilating agents. No cardiovascular lesions were seen in rats following 5 or 13 weeks of administration of Cilostazol at doses up to 1500 mg/kg/day. At this dose, systemic exposures (AUCs) to unbound Cilostazol were only about 1.5 and 5 times (male and female rats, respectively) the exposure seen in humans at the MRHD. Cardiovascular lesions were also not seen in rats following 52 weeks of administration of Cilostazol at doses up to 150 mg/kg/day. At this dose, systemic exposures (AUCs) to unbound Cilostazol were about 0.5 and 5 times (male and female rats, respectively) the exposure in humans at the MRHD. In female rats, Cilostazol AUCs were similar at 150 and 1500 mg/kg/day. Cardiovascular lesions were also not observed in monkeys after oral administration of Cilostazol for 13 weeks at doses up to 1800 mg/kg/day. While this dose of Cilostazol produced pharmacologic effects in monkeys, plasma Cilostazol levels were less than those seen in humans given the MRHD, and those seen in dogs given doses associated with cardiovascular lesions.
Adverse Reactions
Clinical Trials Experience
- Adverse events were assessed in eight placebo-controlled clinical trials involving 2274 patients exposed to either 50 or 100 mg b.i.d. Cilostazol (n=1301) or placebo (n=973), with a median treatment duration of 127 days for patients on Cilostazol and 134 days for patients on placebo.
- The only adverse event resulting in discontinuation of therapy in ≥ 3% of patients treated with Cilostazol 50 or 100 mg b.i.d. was headache, which occurred with an incidence of 1.3%, 3.5%, and 0.3% in patients treated with Cilostazol 50 mg b.i.d., 100 mg b.i.d, or placebo, respectively. Other frequent causes of discontinuation included palpitation and diarrhea, both 1.1% for Cilostazol (all doses) versus 0.1% for placebo.
- The most commonly reported adverse events, occurring in ≥ 2% of patients treated with Cilostazol 50 or 100 mg b.i.d., are shown in the table (below).
- Other events seen with an incidence of ≥ 2%, but occurring in the placebo group at least as frequently as in the 100 mg b.i.d. group were: asthenia, hypertension, vomiting, leg cramps, hypesthesia, paresthesia, dyspnea, rash, hematuria, urinary tract infection, flu syndrome, angina pectoris, arthritis, and bronchitis.
- Less frequent adverse events (<2%) that were experienced by patients exposed to Cilostazol 50 mg b.i.d. or 100 mg b.i.d. in the eight controlled clinical trials and that occurred at a frequency in the 100 mg b.i.d. group greater than in the placebo group, regardless of suspected drug relationship, are listed below.
Body as a Whole
Chills, face edema, fever, generalized edema, malaise, neck rigidity, pelvic pain, retroperitoneal haemorrhage.
Cardiovascular
Atrial fibrillation, atrial flutter, cerebral infarct, cerebral ischemia, congestive heart failure, heart arrest, haemorrhage, hypotension, myocardial infarction, myocardial ischemia, nodal arrhythmia, postural hypotension, supraventricular tachycardia, syncope, varicose vein, vasodilation, ventricular extrasystoles, ventricular tachycardia.
Digestive
Anorexia, cholelithiasis, colitis, duodenal ulcer, duodenitis, esophageal haemorrhage, esophagitis, increased GGT, gastritis, gastroenteritis, gum haemorrhage, hematemesis, melena, peptic ulcer, periodontal abscess, rectal haemorrhage, stomach ulcer, tongue edema.
Endocrine
Diabetes mellitus.
Hemic and Lymphatic
Anemia, ecchymosis, iron deficiency anemia, polycythemia, purpura.
Metabolic and Nutritional
Increased creatinine, gout, hyperlipemia, hyperuricemia.
Musculoskeletal
Arthralgia, bone pain, bursitis.
Nervous
Anxiety, insomnia, neuralgia.
Respiratory
Asthma, epistaxis, hemoptysis, pneumonia, sinusitis.
Skin and Appendages
Dry skin, furunculosis, skin hypertrophy, urticaria.
Special Senses
Amblyopia, blindness, conjunctivitis, diplopia, ear pain, eye haemorrhage, retinal haemorrhage, tinnitus.
Urogenital
Albuminuria, cystitis, urinary frequency, vaginal haemorrhage, vaginitis.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Cilostazol in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cilostazol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cilostazol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Cilostazol with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Cilostazol with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Cilostazol with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Cilostazol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cilostazol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cilostazol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cilostazol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cilostazol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cilostazol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Cilostazol in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Cilostazol in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Cilostazol in the drug label.
Pharmacology
| |
Cilostazol
| |
| Systematic (IUPAC) name | |
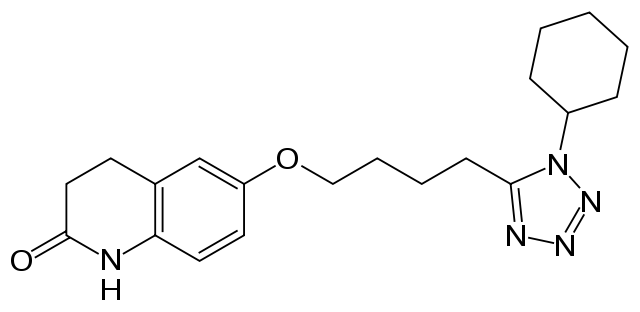
| 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]- 3,4-dihydro-2(1H)-quinolinone | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 369.46 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 95–98% |
| Metabolism | Hepatic (CYP3A4- and CYP2C19-mediated) |
| Half life | 11–13 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Oral |
Mechanism of Action
There is limited information regarding Cilostazol Mechanism of Action in the drug label.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cilostazol in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Cilostazol in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Cilostazol in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Cilostazol in the drug label.
Condition1
- Description
How Supplied
There is limited information regarding Cilostazol How Supplied in the drug label.
Storage
There is limited information regarding Cilostazol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cilostazol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cilostazol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Cilostazol in the drug label.
Precautions with Alcohol
- Alcohol-Cilostazol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Pletal®[3]
Look-Alike Drug Names
- N/A[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Shinoda-Tagawa, T. (2002-02). "A phosphodiesterase inhibitor, Cilostazol, prevents the onset of silent brain infarction in Japanese subjects with Type II diabetes". Diabetologia. 45 (2): 188–194. doi:10.1007/s00125-001-0740-2. ISSN 0012-186X. PMID 11935149. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Ahn, C. W. (2001-04). "Decrease in carotid intima media thickness after 1 year of Cilostazol treatment in patients with type 2 diabetes mellitus". Diabetes Research and Clinical Practice. 52 (1): 45–53. ISSN 0168-8227. PMID 11182215. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "Cilostazol tablet".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Cilostazol |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Cilostazol |Label Name=Cilostazol06.png
}}
{{#subobject:
|Label Page=Cilostazol |Label Name=Cilostazol07.png
}}
{{#subobject:
|Label Page=Cilostazol |Label Name=Cilostazol08.png
}}

