Ceftibuten dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
The recommended doses of CEDAX Oral Suspension are presented in the table below. CEDAX Oral Suspension must be administered at least 2 hours before or 1 hour after a meal.
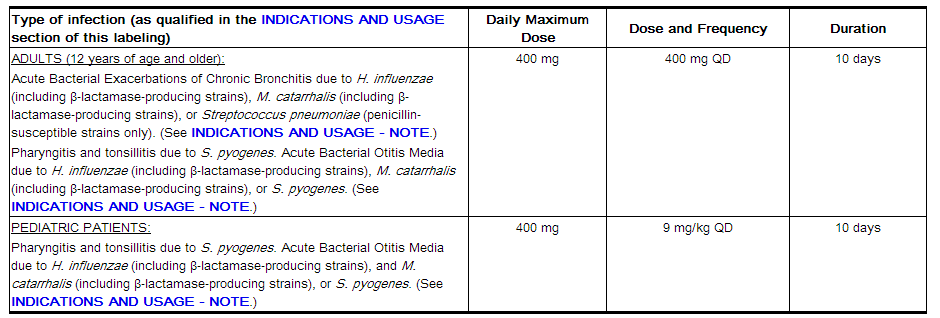
Pediatric patients weighing more than 45 kg should receive the maximum daily dose of 400 mg.
Renal Impairment
CEDAX Capsules and CEDAX Oral Suspension may be administered at normal doses in the presence of impaired renal function with creatinine clearance of 50 mL/min or greater. The recommendations for dosing in patients with varying degrees of renal insufficiency are presented in the following table.
Hemodialysis Patients
In patients undergoing hemodialysis two or three times weekly, a single 400-mg dose of ceftibuten capsules or a single dose of 9 mg/kg (maximum of 400 mg of ceftibuten) oral suspension may be administered at the end of each hemodialysis session.
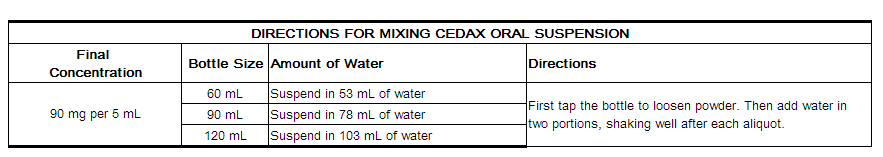
Directions for Mixing CEDAX Oral Suspension
After mixing, the suspension may be kept for 14 days and must be stored in the refrigerator. Keep tightly closed. Shake well before each use. Discard any unused portion after 14 days.
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050686s016lbl.pdf