B-cell lymphoma pathophysiology
|
B-cell lymphoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
B-cell lymphoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of B-cell lymphoma pathophysiology |
|
Risk calculators and risk factors for B-cell lymphoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Shivali Marketkar, M.B.B.S. [2]
Overview
Pathophysiology
Physiology
The normal physiology of B-cells can be understood as follows:
- Development of mature B-cells include:
- B-cells develop from hematopoietic stem cells originating from bone marrow. B-cell development occurs in the bone marrow through several steps including the ordered rearrangement of [[IGH@|Ig H] and L chain loci (i.e., VDJ recombination), positive selection, and negative selection.[1]
- B-cell surface consists of membrane-bound Ig, complement component receptors, Fc receptors, and B cell-specific cell surface molecules representing by CDs (i.e., CD19, CD20, CD21, etc.).
- Immature (transitional) B-cells migrate from the bone marrow to secondary lymphoid organs (i.e., lymph nodes, spleen) for activation. Activation begins with either T-cell dependent or T-cell independent. T-cell-dependent B-cell activation begins with the binding of the B-cell receptor (BCR) to the T-cell-dependent antigen. T-cell independent B-cell activation begins with BCR crosslinking through polysaccharides or via BCR and toll-like receptor (TLR) costimulation. Following the activation, further maturation steps including germinal center reaction (i.e., clonal expansion, class switch recombination, somatic hypermutation, affinity maturation) occur.[2][1]
- B-cells can be divided into 3 main types include: B1 B-lymphoctes (originates from fetal liver), marginal zone (MZ) B2 B-lymphocyes, and follicular (FO) B2 B-lymphocytes.[3]
- B-cells complete their maturation by differention into memory B cells or antibody-secreting plasma cells.
- Functions of B-cells include:[1]
- Antibody production
- Production of cytokines (i.e. IFN-γ, IL-6, IL-10)
- Lymphoid tissue organogenesis
- Wound healing
- Tumor immunity
- Transplant rejection
- Dentritic cell regulation
- TH1/TH2 cytokine balance
- Antigen presentation
- Co-stimulation
Pathogenesis
Genetics
Chromosomal translocations involving the immunoglobulin heavy locus (IGH@) is a classic cytogenetic abnormality for many B-cell lymphomas, including follicular lymphoma, mantle cell lymphoma and Burkitt's lymphoma. In these cases, The immunoglobulin heavy locus forms a fusion protein with another protein that has pro-proliferative or anti-apoptotic abilities. The enhancer element of the immunoglobulin heavy locus, which normally functions to make B cells produce massive production of antibodies, now induces massive transcription of the fusion protein, resulting in excessive pro-proliferative or anti-apoptotic effects on the B cells containing the fusion protein. In Burkitt's lymphoma and mantle cell lymphoma, the other protein in the fusion is c-myc (on chromosome 8) and cyclin D1[4] (on chromosome 11), respectively, which gives the fusion protein pro-proliferative ability. In follicular lymphoma, the fused protein is Bcl-2 (on chromosome 18), which gives the fusion protein anti-apoptotic abilities.
Associated Conditions
Gross Pathology
Microscopic Pathology
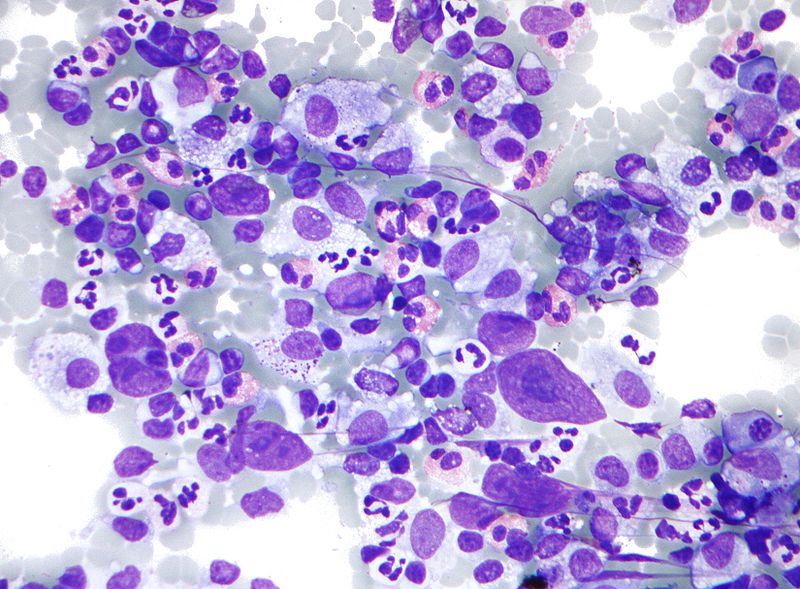
Shown below is a microscopic image of Hodgkins Lymphoma which is a type of B cell lymphoma.Lymph node FNA specimen(Field's stain) The micrograph shows a mixture of cells commonly seen in Hodgkins lymphoma:
- Eosinophils
- Reed Sternberg cells
- Plasma cells
- Histiocytes
Video
Shown below is a video of diffuse large B cell lymphoma {{#ev:youtube|9gEo7si6jtc}}
References
- ↑ 1.0 1.1 1.2 LeBien TW, Tedder TF (September 2008). "B lymphocytes: how they develop and function". Blood. 112 (5): 1570–80. doi:10.1182/blood-2008-02-078071. PMC 2518873. PMID 18725575.
- ↑ Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, Schommartz T, Mertes MM, Schoen CT, Tiburzy B, Herrmann A, Köhl J, Manz RA, Madaio MP, Berger M, Wardemann H, Ehlers M (September 2013). "T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies". J Clin Invest. 123 (9): 3788–96. doi:10.1172/JCI65938. PMC 3754242. PMID 23979161.
- ↑ Hoffman W, Lakkis FG, Chalasani G (January 2016). "B Cells, Antibodies, and More". Clin J Am Soc Nephrol. 11 (1): 137–54. doi:10.2215/CJN.09430915. PMC 4702236. PMID 26700440.
- ↑ Li JY, Gaillard F, Moreau A; et al. (1999). "Detection of translocation t(11;14)(q13;q32) in mantle cell lymphoma by fluorescence in situ hybridization". Am. J. Pathol. 154 (5): 1449–52. doi:10.1016/S0002-9440(10)65399-0. PMC 1866594. PMID 10329598. Unknown parameter
|month=ignored (help)