Autism pathophysiology
|
Autism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Autism pathophysiology On the Web |
|
American Roentgen Ray Society Images of Autism pathophysiology |
|
Risk calculators and risk factors for Autism pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Despite extensive investigation, how autism occurs is not well understood. Its mechanism can be divided into two areas: the pathophysiology of brain structures and processes associated with autism, and the neuropsychological linkages between brain structures and behaviors.[1] The behaviors appear to have multiple pathophysiologies.
Pathophysiology

Autism appears to result from developmental factors that affect many or all functional brain systems,[2] and to disturb the course of brain development more than the final product.[3] Neuroanatomical studies and the associations with teratogens strongly suggest that autism's mechanism includes alteration of brain development soon after conception. This localized anomaly appears to start a cascade of pathological events in the brain that are significantly influenced by environmental factors.[4] Although many major structures of the human brain have been implicated, almost all postmortem studies have been of individuals who also had mental retardation, making it difficult to draw conclusions.[3] Brain weight and volume and head circumference tend to be greater in autistic children.[5] The cellular and molecular bases of pathological early overgrowth are not known, nor is it known whether the overgrown neural systems cause autism's characteristic signs. Current hypotheses include:
- An excess of neurons that causes local overconnectivity in key brain regions.[6]
- Disturbed neuronal migration during early gestation.[7][8]
- Unbalanced excitatory-inhibitory networks.[8]
- Abnormal formation of synapses and dendritic spines.[8]
Interactions between the immune system and the nervous system begin early during embryogenesis, and successful neurodevelopment depends on a balanced immune response. Several symptoms consistent with a poorly regulated immune response have been reported in autistic children. It is possible that aberrant immune activity during critical periods of neurodevelopment is part of the mechanism of some forms of ASD.[9] As autoantibodies have not been associated with pathology, are found in diseases other than ASD, and are not always present in ASD,[10] the relationship between immune disturbances and autism remains unclear and controversial.[7]
Several neurotransmitter abnormalities have been detected in autism, notably increased blood levels of serotonin. Whether these lead to structural or behavioral abnormalities is unclear.[1] Also, some inborn errors of metabolism are associated with autism but probably account for less than 5% of cases.
The mirror neuron system (MNS) theory of autism hypothesizes that distortion in the development of the MNS interferes with imitation and leads to autism's core features of social impairment and communication difficulties. The MNS operates when an animal performs an action or observes another animal of the same species perform the same action. The MNS may contribute to an individual's understanding of other people by enabling the modeling of their behavior via embodied simulation of their actions, intentions, and emotions.[11] Several studies have tested this hypothesis by demonstrating structural abnormalities in MNS regions of individuals with ASD, delay in the activation in the core circuit for imitation in individuals with Asperger's, and a correlation between reduced MNS activity and severity of the syndrome in children with ASD.[12] However, individuals with autism also have abnormal brain activation in many circuits outside the MNS[13] and the MNS theory does not explain the normal performance of autistic children on imitation tasks that involve a goal or object.[14]
A 2008 study of autistic adults found evidence for altered functional organization of the task-negative network, a large-scale brain network involved in social and emotional processing, with intact organization of the task-positive network, used in sustained attention and goal-directed thinking.[15] A 2008 brain-imaging study found a specific pattern of signals in the cingulate cortex which differs in individuals with ASD.[16]
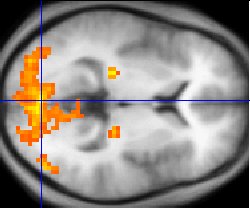
The underconnectivity theory of autism hypothesizes that autism is marked by underfunctioning high-level neural connections and synchronization, along with an excess of low-level processes.[17] Evidence for this theory has been found in functional neuroimaging studies on autistic individuals[18] and by a brain wave study that suggested that adults with ASD have local overconnectivity in the cortex and weak functional connections between the frontal lobe and the rest of the cortex.[19] Other evidence suggests the underconnectivity is mainly within each hemisphere of the cortex and that autism is a disorder of the association cortex.[20]
Neuropsychology
Two major categories of cognitive theories have been proposed about the links between autistic brains and behavior.
The first category focuses on deficits in social cognition. Hyper-systemizing hypothesizes that autistic individuals can systematize—that is, they can develop internal rules of operation to handle internal events—but are less effective at empathizing by handling events generated by other agents.[21] It extends the extreme male brain theory, which hypothesizes that autism is an extreme case of the male brain, defined psychometrically as individuals in whom systemizing is better than empathizing.[22] This in turn is related to the earlier theory of mind, which hypothesizes that autistic behavior arises from an inability to ascribe mental states to oneself and others. The theory of mind is supported by autistic children's atypical responses to the Sally-Anne test for reasoning about others' motivations,[23] and is mapped well from the mirror neuron system theory of autism.[12]
The second category focuses on nonsocial or general processing. Executive dysfunction hypothesizes that autistic behavior results in part from deficits in flexibility, planning, and other forms of executive function. A strength of the theory is predicting stereotyped behavior and narrow interests;[24] a weakness is that executive function deficits are not found in young autistic children. Weak central coherence theory hypothesizes that a limited ability to see the big picture underlies the central disturbance in autism. One strength of this theory is predicting special talents and peaks in performance in autistic people.[25] A related theory—enhanced perceptual functioning—focuses more on the superiority of locally oriented and perceptual operations in autistic individuals.[26] These theories map well from the underconnectivity theory of autism.
Neither category is satisfactory on its own; social cognition theories poorly address autism's rigid and repetitive behaviors, while the nonsocial theories have difficulty explaining social impairment and communication difficulties.[27] A combined theory based on multiple deficits may prove to be more useful.[28]
References
- ↑ 1.0 1.1 Penn HE (2006). "Neurobiological correlates of autism: a review of recent research". Child Neuropsychol. 12 (1): 57–79. doi:10.1080/09297040500253546. PMID 16484102.
- ↑ Müller RA (2007). "The study of autism as a distributed disorder". Ment Retard Dev Disabil Res Rev. 13 (1): 85–95. doi:10.1002/mrdd.20141. PMID 17326118.
- ↑ 3.0 3.1 Amaral DG, Schumann CM, Nordahl CW (2008). "Neuroanatomy of autism". Trends Neurosci. 31 (3): 137–45. doi:10.1016/j.tins.2007.12.005. PMID 18258309.
- ↑ Casanova MF (2007). "The neuropathology of autism". Brain Pathol. 17 (4): 422–33. doi:10.1111/j.1750-3639.2007.00100.x. PMID 17919128.
- ↑ DiCicco-Bloom E, Lord C, Zwaigenbaum L; et al. (2006). "The developmental neurobiology of autism spectrum disorder". J Neurosci. 26 (26): 6897–906. doi:10.1523/JNEUROSCI.1712-06.2006. PMID 16807320.
- ↑ Courchesne E, Pierce K, Schumann CM; et al. (2007). "Mapping early brain development in autism". Neuron. 56 (2): 399–413. doi:10.1016/j.neuron.2007.10.016. PMID 17964254.
- ↑ 7.0 7.1 Schmitz C, Rezaie P (2008). "The neuropathology of autism: where do we stand?". Neuropathol Appl Neurobiol. 34 (1): 4–11. doi:10.1111/j.1365-2990.2007.00872.x. PMID 17971078.
- ↑ 8.0 8.1 8.2 Persico AM, Bourgeron T (2006). "Searching for ways out of the autism maze: genetic, epigenetic and environmental clues". Trends Neurosci. 29 (7): 349–58. doi:10.1016/j.tins.2006.05.010. PMID 16808981.
- ↑ Ashwood P, Wills S, Van de Water J (2006). "The immune response in autism: a new frontier for autism research". J Leukoc Biol. 80 (1): 1–15. doi:10.1189/jlb.1205707. PMID 16698940.
- ↑ Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J (2007). "Autoantibodies in autism spectrum disorders (ASD)". Ann N Y Acad Sci. 1107: 79–91. doi:10.1196/annals.1381.009. PMID 17804535.
- ↑ MNS and autism:
- Ramachandran VS, Oberman LM (2006). "Broken mirrors: a theory of autism" (PDF). Sci Am. 295 (5): 62–9. PMID 17076085. Retrieved 2008-04-17.
- Dinstein I, Thomas C, Behrmann M, Heeger DJ (2008). "A mirror up to nature". Curr Biol. 18 (1): R13–8. doi:10.1016/j.cub.2007.11.004. PMID 18177704.
- ↑ 12.0 12.1 Iacoboni M, Dapretto M (2006). "The mirror neuron system and the consequences of its dysfunction". Nat Rev Neurosci. 7 (12): 942–51. doi:10.1038/nrn2024. PMID 17115076.
- ↑ Frith U, Frith CD (2003). "Development and neurophysiology of mentalizing" (PDF). Philos Trans R Soc Lond B Biol Sci. 358 (1431): 459–73. doi:10.1098/rstb.2002.1218. PMID 12689373.
- ↑ Hamilton AFdC (2008). "Emulation and mimicry for social interaction: a theoretical approach to imitation in autism". Q J Exp Psychol. 61 (1): 101–15. doi:10.1080/17470210701508798. PMID 18038342.
- ↑ Kennedy DP, Courchesne E (2008). "The intrinsic functional organization of the brain is altered in autism". Neuroimage. 38 (4): 1877–85. doi:10.1016/j.neuroimage.2007.10.052. PMID 18083565.
- ↑ Chiu PH, Kayali MA, Kishida KT; et al. (2008). "Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism". Neuron. 57 (3): 463–73. doi:10.1016/j.neuron.2007.12.020. PMID 18255038. Lay summary – Technol Rev (2007-02-07).
- ↑ Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ (2007). "Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry". Cereb Cortex. 17 (4): 951–61. doi:10.1093/cercor/bhl006. PMID 16772313.
- ↑ Williams DL, Goldstein G, Minshew NJ (2006). "Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing". Child Neuropsychol. 12 (4–5): 279–98. doi:10.1080/09297040600681190. PMC 1803025. PMID 16911973.
- ↑ Murias M, Webb SJ, Greenson J, Dawson G (2007). "Resting state cortical connectivity reflected in EEG coherence in individuals with autism". Biol Psychiatry. 62 (3): 270–3. doi:10.1016/j.biopsych.2006.11.012. PMID 17336944.
- ↑ Minshew NJ, Williams DL (2007). "The new neurobiology of autism: cortex, connectivity, and neuronal organization". Arch Neurol. 64 (7): 945–50. PMID 17620483.
- ↑ Baron-Cohen S (2006). "The hyper-systemizing, assortative mating theory of autism". Prog Neuropsychopharmacol Biol Psychiatry. 30 (5): 865–72. doi:10.1016/j.pnpbp.2006.01.010. PMID 16519981.
- ↑ Baron-Cohen S (2002). "The extreme male brain theory of autism". Trends Cogn Sci. 6 (6): 248–54. doi:10.1016/S1364-6613(02)01904-6. PMID 12039606.
- ↑ Baron-Cohen S, Leslie AM, Frith U (1985). "Does the autistic child have a 'theory of mind'?" (PDF). Cognition. 21 (1): 37–46. doi:10.1016/0010-0277(85)90022-8. PMID 2934210. Retrieved 2007-06-28.
- ↑ Hill EL (2004). "Executive dysfunction in autism". Trends Cogn Sci. 8 (1): 26–32. doi:10.1016/j.dr.2004.01.001. PMID 14697400.
- ↑ Happé F, Frith U (2006). "The weak coherence account: detail-focused cognitive style in autism spectrum disorders". J Autism Dev Disord. 36 (1): 5–25. doi:10.1007/s10803-005-0039-0. PMID 16450045.
- ↑ Mottron L, Dawson M, Soulières I, Hubert B, Burack J (2006). "Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception". J Autism Dev Disord. 36 (1): 27–43. doi:10.1007/s10803-005-0040-7. PMID 16453071.
- ↑ Happé F, Ronald A, Plomin R (2006). "Time to give up on a single explanation for autism". Nat Neurosci. 9 (10): 1218–20. doi:10.1038/nn1770. PMID 17001340.
- ↑ Rajendran G, Mitchell P (2007). "Cognitive theories of autism". Dev Rev. 27 (2): 224–60. doi:10.1016/j.dr.2007.02.001.