Aripiprazole (intramuscular)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDALITY AND ANTIDEPRESSANT DRUGS
See full prescribing information for complete Boxed Warning.
* Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis.
|
Overview
Aripiprazole (intramuscular) is an atypical antipsychotic that is FDA approved for the treatment of schizophrenia, bipolar I disorder, major depressive disorder, and irritability associated with autistic disorder. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, vomiting, constipation, headache, dizziness, akathisia, anxiety, insomnia, and restlessness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Schizophrenia
- Dosing Information
- The recommended starting and target dose for ABILIFY is 10 mg/day or 15 mg/day administered on a once-a-day schedule without regard to meals.
- Maintenance Treatment: Maintenance of efficacy in schizophrenia was demonstrated in a trial involving patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from those medications and randomized to either ABILIFY 15 mg/day or placebo, and observed for relapse. Patients should be periodically reassessed to determine the continued need for maintenance treatment.
Bipolar I Disorder
- Dosing Information
- Acute Treatment of Manic and Mixed Episodes
- ABILIFY is indicated for the acute treatment of manic and mixed episodes associated with bipolar I disorder, both as monotherapy and as an adjunct to lithium or valproate. Efficacy as monotherapy was established in four 3-week monotherapy trials in adults and one 4-week monotherapy trial in pediatric patients (10 to 17 years). Efficacy as adjunctive therapy was established in one 6-week adjunctive trial in adults .
- Maintenance Treatment of Bipolar I Disorder
- ABILIFY is indicated for the maintenance treatment of bipolar I disorder, both as monotherapy and as an adjunct to either lithium or valproate. Maintenance efficacy was demonstrated in one monotherapy maintenance trial and in one adjunctive maintenance trial in adults .
Adjunctive Treatment of Major Depressive Disorder
- Dosing Information
- ABILIFY is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder (MDD). Efficacy was established in two 6-week trials in adults with MDD who had an inadequate response to antidepressant therapy during the current episode.
Irritability Associated with Autistic Disorder
- Dosing Information
- ABILIFY is indicated for the treatment of irritability associated with autistic disorder. Efficacy was established in two 8-week trials in pediatric patients (aged 6 to 17 years) with irritability associated with autistic disorder (including symptoms of aggression towards others, deliberate self-injuriousness, temper tantrums, and quickly changing moods) .
Agitation Associated with Schizophrenia or Bipolar Mania (Intramuscular Injection)
- Dosing Information
- ABILIFY Injection is indicated for the acute treatment of agitation associated with schizophrenia or bipolar disorder, manic or mixed. “Psychomotor agitation” is defined in DSM-IV as “excessive motor activity associated with a feeling of inner tension.” Patients experiencing agitation often manifest behaviors that interfere with their diagnosis and care (eg, threatening behaviors, escalating or urgently distressing behavior, or self-exhausting behavior), leading clinicians to the use of intramuscular antipsychotic medications to achieve immediate control of the agitation. Efficacy was established in three short-term (24-hour) trials in adults .
Dosage Adjustment
Dosage adjustments in adults are not routinely indicated on the basis of age, gender, race, or renal or hepatic impairment status.
- Dosage adjustment for patients taking aripiprazole concomitantly with strong CYP3A4 inhibitors: When concomitant administration of aripiprazole with strong CYP3A4 inhibitors such as ketoconazole or clarithromycin is indicated, the aripiprazole dose should be reduced to one-half of the usual dose. When the CYP3A4 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should then be increased.
- Dosage adjustment for patients taking aripiprazole concomitantly with potential CYP2D6 inhibitors: When concomitant administration of potential CYP2D6 inhibitors such as quinidine, fluoxetine, or paroxetine with aripiprazole occurs, aripiprazole dose should be reduced at least to one-half of its normal dose. When the CYP2D6 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should then be increased. When adjunctive ABILIFY is administered to patients with major depressive disorder, ABILIFY should be administered without dosage adjustment.
- Dosing recommendation in patients taking aripiprazole concomitantly with strong CYP3A4 and CYP2D6 inhibitors: When concomitant administration of aripiprazole with strong inhibitors of CYP3A4 (such as ketoconazole or clarithromycin) and CYP2D6 (such as quinidine, fluoxetine, or paroxetine) is indicated, the aripiprazole dose should be reduced to one-quarter (25%) of the usual dose. When the CYP3A4 and/or CYP2D6 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should be increased.
- Dosing recommendation in patients taking aripiprazole concomitantly with strong, moderate, or weak inhibitors of CYP3A4 and CYP2D6: Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (eg, a potent CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favorable clinical response.
- Dosing recommendation in patients who are classified as CYP2D6 poor metabolizers (PM): The aripiprazole dose in PM patients should initially be reduced to one-half (50%) of the usual dose and then adjusted to achieve a favorable clinical response. The dose of aripiprazole for PM patients who are administered a strong CYP3A4 inhibitor should be reduced to one-quarter (25%) of the usual dose.
- Dosage adjustment for patients taking potential CYP3A4 inducers: When a potential CYP3A4 inducer such as carbamazepine is added to aripiprazole therapy, the aripiprazole dose should be doubled. Additional dose increases should be based on clinical evaluation. When the CYP3A4 inducer is withdrawn from the combination therapy, the aripiprazole dose should be reduced to 10 mg to 15 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aripiprazole in adult patients.
Non–Guideline-Supported Use
Borderline personality disorder
- Dosing Information
- Aripiprazole 10-15 mg/day added to sertraline 100-200 mg/day[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about the guideline-supported off label used in pediatric patients.
Non–Guideline-Supported Use
There is limited information about the non-guideline-supported off label used in pediatric patients.
Contraindications
- Known hypersensitivity reaction to ABILIFY. Reactions have ranged from pruritus/urticaria to anaphylaxis.
Warnings
|
WARNINGS: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDALITY AND ANTIDEPRESSANT DRUGS
See full prescribing information for complete Boxed Warning.
* Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis.
|
Use in Elderly Patients with Dementia-Related Psychosis
Increased Mortality
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis
Cerebrovascular Adverse Events, Including Stroke
In placebo-controlled clinical studies (two flexible dose and one fixed dose study) of dementia-related psychosis, there was an increased incidence of cerebrovascular adverse events (eg, stroke, transient ischemic attack), including fatalities, in aripiprazole-treated patients (mean age: 84 years; range: 78-88 years). In the fixed-dose study, there was a statistically significant dose response relationship for cerebrovascular adverse events in patients treated with aripiprazole. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis.
Safety Experience in Elderly Patients with Psychosis Associated with Alzheimer's Disease
- In three, 10-week, placebo-controlled studies of aripiprazole in elderly patients with psychosis associated with Alzheimer's disease (n=938; mean age: 82.4 years; range: 56-99 years), the treatment-emergent adverse events that were reported at an incidence of ≥3% and aripiprazole incidence at least twice that for placebo were lethargy [placebo 2%, aripiprazole 5%], somnolence (including sedation) [placebo 3%, aripiprazole 8%], and incontinence (primarily, urinary incontinence) [placebo 1%, aripiprazole 5%], excessive salivation [placebo 0%, aripiprazole 4%], and lightheadedness [placebo 1%, aripiprazole 4%].
- The safety and efficacy of ABILIFY in the treatment of patients with psychosis associated with dementia have not been established. If the prescriber elects to treat such patients with ABILIFY, vigilance should be exercised, particularly for the emergence of difficulty swallowing or excessive somnolence, which could predispose to accidental injury or aspiration.
Clinical Worsening of Depression and Suicide Risk
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term, placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with MDD and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
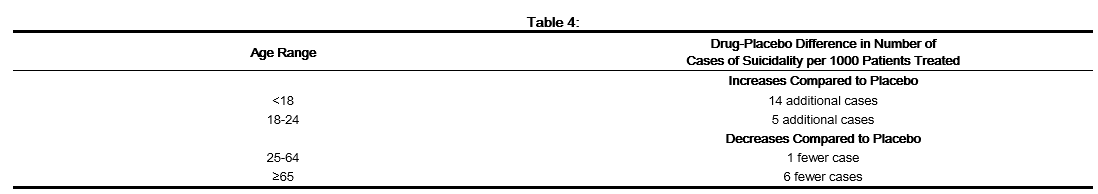
- The pooled analyses of placebo-controlled trials in children and adolescents with MDD, Obsessive Compulsive Disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 4.
Adverse Reactions
Clinical Trials Experience
Overall Adverse Reactions Profile
- The following are discussed in more detail in other sections of the labeling:
- Use in Elderly Patients with Dementia-Related Psychosis
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults
- Neuroleptic Malignant Syndrome (NMS)
- Tardive Dyskinesia
- Metabolic Changes
- Orthostatic Hypotension
- Leukopenia, Neutropenia, and Agranulocytosis
- Seizures/Convulsions
- Potential for Cognitive and Motor Impairment
- Body Temperature Regulation
- Suicide
- Dysphagia
- Use in Patients with Concomitant Illness
- The most common adverse reactions in adult patients in clinical trials (≥10%) were nausea, vomiting, constipation, headache, dizziness, akathisia, anxiety, insomnia, and restlessness.
- The most common adverse reactions in the pediatric clinical trials (≥10%) were somnolence, headache, vomiting, extrapyramidal disorder, fatigue, increased appetite, insomnia, nausea, nasopharyngitis, and weight increased.
- Aripiprazole has been evaluated for safety in 13,543 adult patients who participated in multiple-dose, clinical trials in schizophrenia, bipolar disorder, major depressive disorder, Dementia of the Alzheimer’s type, Parkinson’s disease, and alcoholism, and who had approximately 7619 patient-years of exposure to oral aripiprazole and 749 patients with exposure to aripiprazole injection. A total of 3390 patients were treated with oral aripiprazole for at least 180 days and 1933 patients treated with oral aripiprazole had at least 1 year of exposure.
- Aripiprazole has been evaluated for safety in 920 patients (6 to 17 years) who participated in multiple-dose, clinical trials in schizophrenia, bipolar mania, or autistic disorder and who had approximately 517 patient-years of exposure to oral aripiprazole. A total of 465 pediatric patients were treated with oral aripiprazole for at least 180 days and 117 pediatric patients treated with oral aripiprazole had at least 1 year of exposure.
- The conditions and duration of treatment with aripiprazole (monotherapy and adjunctive therapy with antidepressants or mood stabilizers) included (in overlapping categories) double-blind, comparative and noncomparative open-label studies, inpatient and outpatient studies, fixed- and flexible-dose studies, and short- and longer-term exposure.
- Adverse events during exposure were obtained by collecting volunteered adverse events, as well as results of physical examinations, vital signs, weights, laboratory analyses, and ECG. Adverse experiences were recorded by clinical investigators using terminology of their own choosing. In the tables and tabulations that follow, MedDRA dictionary terminology has been used to classify reported adverse events into a smaller number of standardized event categories, in order to provide a meaningful estimate of the proportion of individuals experiencing adverse events.
- The stated frequencies of adverse reactions represent the proportion of individuals who experienced at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation. There was no attempt to use investigator causality assessments; ie, all events meeting the defined criteria, regardless of investigator causality, are included.
- Throughout this section, adverse reactions are reported. These are adverse events that were considered to be reasonably associated with the use of ABILIFY (adverse drug reactions) based on the comprehensive assessment of the available adverse event information. A causal association for ABILIFY often cannot be reliably established in individual cases.
- The figures in the tables and tabulations cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those that prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatment, uses, and investigators. The cited figures, however, do provide the prescriber with some basis for estimating the relative contribution of drug and nondrug factors to the adverse reaction incidence in the population studied.
Clinical Studies Experience
Adult Patients with Schizophrenia
- The following findings are based on a pool of five placebo-controlled trials (four 4-week and one 6-week) in which oral aripiprazole was administered in doses ranging from 2 mg/day to 30 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
- Overall, there was little difference in the incidence of discontinuation due to adverse reactions between aripiprazole-treated (7%) and placebo-treated (9%) patients. The types of adverse reactions that led to discontinuation were similar for the aripiprazole-treated and placebo-treated patients.
Commonly Observed Adverse Reactions
- The only commonly observed adverse reaction associated with the use of aripiprazole in patients with schizophrenia (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) was akathisia (aripiprazole 8%; placebo 4%).
Adult Patients with Bipolar Mania
- Monotherapy
- The following findings are based on a pool of 3-week, placebo-controlled, bipolar mania trials in which oral aripiprazole was administered at doses of 15 mg/day or 30 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
- Overall, in patients with bipolar mania, there was little difference in the incidence of discontinuation due to adverse reactions between aripiprazole-treated (11%) and placebo-treated (10%) patients. The types of adverse reactions that led to discontinuation were similar between the aripiprazole-treated and placebo-treated patients.
Commonly Observed Adverse Reactions
- Commonly observed adverse reactions associated with the use of aripiprazole in patients with bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) are shown in Table 14.
Table 14:
Less Common Adverse Reactions in Adults
- Table 15 enumerates the pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia and up to 3 weeks in bipolar mania), including only those reactions that occurred in 2% or more of patients treated with aripiprazole (doses ≥2 mg/day) and for which the incidence in patients treated with aripiprazole was greater than the incidence in patients treated with placebo in the combined dataset.
- An examination of population subgroups did not reveal any clear evidence of differential adverse reaction incidence on the basis of age, gender, or race.
Adult Patients with Adjunctive Therapy with Bipolar Mania
- The following findings are based on a placebo-controlled trial of adult patients with bipolar disorder in which aripiprazole was administered at doses of 15 mg/day or 30 mg/day as adjunctive therapy with lithium or valproate.
Adverse Reactions Associated with Discontinuation of Treatment
- In a study of patients who were already tolerating either lithium or valproate as monotherapy, discontinuation rates due to adverse reactions were 12% for patients treated with adjunctive aripiprazole compared to 6% for patients treated with adjunctive placebo. The most common adverse drug reactions associated with discontinuation in the adjunctive aripiprazole-treated compared to placebo-treated patients were akathisia (5% and 1%, respectively) and tremor (2% and 1%, respectively).
Commonly Observed Adverse Reactions
- The commonly observed adverse reactions associated with adjunctive aripiprazole and lithium or valproate in patients with bipolar mania (incidence of 5% or greater and incidence at least twice that for adjunctive placebo) were: akathisia, insomnia, and extrapyramidal disorder.
Less Common Adverse Reactions in Adult Patients with Adjunctive Therapy in Bipolar Mania
- Table 16 enumerates the incidence, rounded to the nearest percent, of adverse reactions that occurred during acute treatment (up to 6 weeks), including only those reactions that occurred in 2% or more of patients treated with adjunctive aripiprazole (doses of 15 mg/day or 30 mg/day) and lithium or valproate and for which the incidence in patients treated with this combination was greater than the incidence in patients treated with placebo plus lithium or valproate.
Pediatric Patients (13 to 17 years) with Schizophrenia
- The following findings are based on one 6-week, placebo-controlled trial in which oral aripiprazole was administered in doses ranging from 2 mg/day to 30 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
- The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (13 to 17 years) was 5% and 2%, respectively.
Commonly Observed Adverse Reactions
- Commonly observed adverse reactions associated with the use of aripiprazole in adolescent patients with schizophrenia (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) were extrapyramidal disorder, somnolence, and tremor.
Pediatric Patients (10 to 17 years) with Bipolar Mania
- The following findings are based on one 4-week, placebo-controlled trial in which oral aripiprazole was administered in doses of 10 mg/day or 30 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
- The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (10 to 17 years) was 7% and 2%, respectively.
Commonly Observed Adverse Reactions
- Commonly observed adverse reactions associated with the use of aripiprazole in pediatric patients with bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) are shown in Table 17.
Pediatric Patients (6 to 17 years) with Autistic Disorder
- The following findings are based on two 8-week, placebo-controlled trials in which oral aripiprazole was administered in doses of 2 mg/day to 15 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
- The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (6 to 17 years) was 10% and 8%, respectively.
Commonly Observed Adverse Reactions
- Commonly observed adverse reactions associated with the use of aripiprazole in pediatric patients with autistic disorder (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) are shown in Table 18.
Less Common Adverse Reactions in Pediatric Patients (6 to 17 years) with Schizophrenia, Bipolar Mania, or Autistic Disorder
- Table 19 enumerates the pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia, up to 4 weeks in bipolar mania, and up to 8 weeks in autistic disorder), including only those reactions that occurred in 1% or more of pediatric patients treated with aripiprazole (doses ≥2 mg/day) and for which the incidence in patients treated with aripiprazole was greater than the incidence in patients treated with placebo.
Adult Patients Receiving ABILIFY as Adjunctive Treatment of Major Depressive Disorder
- The following findings are based on a pool of two placebo-controlled trials of patients with major depressive disorder in which aripiprazole was administered at doses of 2 mg to 20 mg as adjunctive treatment to continued antidepressant therapy.
Adverse Reactions Associated with Discontinuation of Treatment
- The incidence of discontinuation due to adverse reactions was 6% for adjunctive aripiprazole-treated patients and 2% for adjunctive placebo-treated patients.
Commonly Observed Adverse Reactions
- The commonly observed adverse reactions associated with the use of adjunctive aripiprazole in patients with major depressive disorder (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) were: akathisia, restlessness, insomnia, constipation, fatigue, and blurred vision.
Less Common Adverse Reactions in Adult Patients with Major Depressive Disorder
- Table 20 enumerates the pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks), including only those adverse reactions that occurred in 2% or more of patients treated with adjunctive aripiprazole (doses ≥2 mg/day) and for which the incidence in patients treated with adjunctive aripiprazole was greater than the incidence in patients treated with adjunctive placebo in the combined dataset.
Patients with Agitation Associated with Schizophrenia or Bipolar Mania (Intramuscular Injection)
- The following findings are based on a pool of three placebo-controlled trials of patients with agitation associated with schizophrenia or bipolar mania in which aripiprazole injection was administered at doses of 5.25 mg to 15 mg.
Adverse Reactions Associated with Discontinuation of Treatment
- Overall, in patients with agitation associated with schizophrenia or bipolar mania, there was little difference in the incidence of discontinuation due to adverse reactions between aripiprazole-treated (0.8%) and placebo-treated (0.5%) patients.
Commonly Observed Adverse Reactions
- There was one commonly observed adverse reaction (nausea) associated with the use of aripiprazole injection in patients with agitation associated with schizophrenia and bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo).
Less Common Adverse Reactions in Patients with Agitation Associated with Schizophrenia or Bipolar Mania
- Table 21 enumerates the pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (24-hour), including only those adverse reactions that occurred in 2% or more of patients treated with aripiprazole injection (doses ≥5.25 mg/day) and for which the incidence in patients treated with aripiprazole injection was greater than the incidence in patients treated with placebo in the combined dataset.
Dose-Related Adverse Reactions
Schizophrenia
- Dose response relationships for the incidence of treatment-emergent adverse events were evaluated from four trials in adult patients with schizophrenia comparing various fixed doses (2 mg/day, 5 mg/day, 10 mg/day, 15 mg/day, 20 mg/day, and 30 mg/day) of oral aripiprazole to placebo. This analysis, stratified by study, indicated that the only adverse reaction to have a possible dose response relationship, and then most prominent only with 30 mg, was somnolence [including sedation]; (incidences were placebo, 7.1%; 10 mg, 8.5%; 15 mg, 8.7%; 20 mg, 7.5%; 30 mg, 12.6%).
- In the study of pediatric patients (13 to 17 years of age) with schizophrenia, three common adverse reactions appeared to have a possible dose response relationship: extrapyramidal disorder (incidences were placebo, 5.0%; 10 mg, 13.0%; 30 mg, 21.6%); somnolence (incidences were placebo, 6.0%; 10 mg, 11.0%; 30 mg, 21.6%); and tremor (incidences were placebo, 2.0%; 10 mg, 2.0%; 30 mg, 11.8%).
Bipolar Mania
- In the study of pediatric patients (10 to 17 years of age) with bipolar mania, four common adverse reactions had a possible dose response relationship at 4 weeks; extrapyramidal disorder (incidences were placebo, 3.1%; 10 mg, 12.2%; 30 mg, 27.3%); somnolence (incidences were placebo, 3.1%; 10 mg, 19.4%; 30 mg, 26.3%); akathisia (incidences were placebo, 2.1%; 10 mg, 8.2%; 30 mg, 11.1%); and salivary hypersecretion (incidences were placebo, 0%; 10 mg, 3.1%; 30 mg, 8.1%).
Autistic Disorder
- In a study of pediatric patients (6 to 17 years of age) with autistic disorder, one common adverse reaction had a possible dose response relationship: fatigue (incidences were placebo, 0%; 5 mg, 3.8%; 10 mg, 22.0%; 15 mg, 18.5%).
Extrapyramidal Symptoms
Schizophrenia
- In short-term, placebo-controlled trials in schizophrenia in adults, the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 13% vs. 12% for placebo; and the incidence of akathisia-related events for aripiprazole-treated patients was 8% vs. 4% for placebo. In the short-term, placebo-controlled trial of schizophrenia in pediatric patients (13 to 17 years), the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 25% vs. 7% for placebo; and the incidence of akathisia-related events for aripiprazole-treated patients was 9% vs. 6% for placebo.
- Objectively collected data from those trials was collected on the Simpson Angus Rating Scale (for EPS), the Barnes Akathisia Scale (for akathisia), and the Assessments of Involuntary Movement Scales (for dyskinesias). In the adult schizophrenia trials, the objectively collected data did not show a difference between aripiprazole and placebo, with the exception of the Barnes Akathisia Scale (aripiprazole, 0.08; placebo, −0.05). In the pediatric (13 to 17 years) schizophrenia trial, the objectively collected data did not show a difference between aripiprazole and placebo, with the exception of the Simpson Angus Rating Scale (aripiprazole, 0.24; placebo, −0.29).
- Similarly, in a long-term (26-week), placebo-controlled trial of schizophrenia in adults, objectively collected data on the Simpson Angus Rating Scale (for EPS), the Barnes Akathisia Scale (for akathisia), and the Assessments of Involuntary Movement Scales (for dyskinesias) did not show a difference between aripiprazole and placebo.
Bipolar Mania
- In the short-term, placebo-controlled trials in bipolar mania in adults, the incidence of reported EPS-related events, excluding events related to akathisia, for monotherapy aripiprazole-treated patients was 16% vs. 8% for placebo and the incidence of akathisia-related events for monotherapy aripiprazole-treated patients was 13% vs. 4% for placebo. In the 6-week, placebo-controlled trial in bipolar mania for adjunctive therapy with lithium or valproate, the incidence of reported EPS-related events, excluding events related to akathisia for adjunctive aripiprazole-treated patients was 15% vs. 8% for adjunctive placebo and the incidence of akathisia-related events for adjunctive aripiprazole-treated patients was 19% vs. 5% for adjunctive placebo. In the short-term, placebo-controlled trial in bipolar mania in pediatric (10 to 17 years) patients, the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 26% vs. 5% for placebo and the incidence of akathisia-related events for aripiprazole-treated patients was 10% vs. 2% for placebo.
- In the adult bipolar mania trials with monotherapy aripiprazole, the Simpson Angus Rating Scale and the Barnes Akathisia Scale showed a significant difference between aripiprazole and placebo (aripiprazole, 0.50; placebo, −0.01 and aripiprazole, 0.21; placebo, −0.05). Changes in the Assessments of Involuntary Movement Scales were similar for the aripiprazole and placebo groups. In the bipolar mania trials with aripiprazole as adjunctive therapy with either lithium or valproate, the Simpson Angus Rating Scale and the Barnes Akathisia Scale showed a significant difference between adjunctive aripiprazole and adjunctive placebo (aripiprazole, 0.73; placebo, 0.07 and aripiprazole, 0.30; placebo, 0.11). Changes in the Assessments of Involuntary Movement Scales were similar for adjunctive aripiprazole and adjunctive placebo. In the pediatric (10 to 17 years), short-term, bipolar mania trial, the Simpson Angus Rating Scale showed a significant difference between aripiprazole and placebo (aripiprazole, 0.90; placebo, –0.05). Changes in the Barnes Akathisia Scale and the Assessments of Involuntary Movement Scales were similar for the aripiprazole and placebo groups.
Major Depressive Disorder
- In the short-term, placebo-controlled trials in major depressive disorder, the incidence of reported EPS-related events, excluding events related to akathisia, for adjunctive aripiprazole-treated patients was 8% vs. 5% for adjunctive placebo-treated patients; and the incidence of akathisia-related events for adjunctive aripiprazole-treated patients was 25% vs. 4% for adjunctive placebo-treated patients.
- In the major depressive disorder trials, the Simpson Angus Rating Scale and the Barnes Akathisia Scale showed a significant difference between adjunctive aripiprazole and adjunctive placebo (aripiprazole, 0.31; placebo, 0.03 and aripiprazole, 0.22; placebo, 0.02). Changes in the Assessments of Involuntary Movement Scales were similar for the adjunctive aripiprazole and adjunctive placebo groups.
Autistic Disorder
- In the short-term, placebo-controlled trials in autistic disorder in pediatric patients (6 to 17 years), the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 18% vs. 2% for placebo and the incidence of akathisia-related events for aripiprazole-treated patients was 3% vs. 9% for placebo.
- In the pediatric (6 to 17 years) short-term autistic disorder trials, the Simpson Angus Rating Scale showed a significant difference between aripiprazole and placebo (aripiprazole, 0.1; placebo, −0.4). Changes in the Barnes Akathisia Scale and the Assessments of Involuntary Movement Scales were similar for the aripiprazole and placebo groups.
Agitation Associated with Schizophrenia or Bipolar Mania
- In the placebo-controlled trials in patients with agitation associated with schizophrenia or bipolar mania, the incidence of reported EPS-related events excluding events related to akathisia for aripiprazole-treated patients was 2% vs. 2% for placebo and the incidence of akathisia-related events for aripiprazole-treated patients was 2% vs. 0% for placebo. Objectively collected data on the Simpson Angus Rating Scale (for EPS) and the Barnes Akathisia Scale (for akathisia) for all treatment groups did not show a difference between aripiprazole and placebo.
Dystonia
- Class Effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Laboratory Test Abnormalities
- A between group comparison for 3-week to 6-week, placebo-controlled trials in adults or 4-week to 8-week, placebo-controlled trials in pediatric patients (6 to 17 years) revealed no medically important differences between the aripiprazole and placebo groups in the proportions of patients experiencing potentially clinically significant changes in routine serum chemistry, hematology, or urinalysis parameters. Similarly, there were no aripiprazole/placebo differences in the incidence of discontinuations for changes in serum chemistry, hematology, or urinalysis in adult or pediatric patients.
ECG Changes
- Between group comparisons for a pooled analysis of placebo-controlled trials in patients with schizophrenia, bipolar mania, or major depressive disorder revealed no significant differences between oral aripiprazole and placebo in the proportion of patients experiencing potentially important changes in ECG parameters. Aripiprazole was associated with a median increase in heart rate of 2 beats per minute compared to no increase among placebo patients.
- In the pooled, placebo-controlled trials in patients with agitation associated with schizophrenia or bipolar mania, there were no significant differences between aripiprazole injection and placebo in the proportion of patients experiencing potentially important changes in ECG parameters, as measured by standard 12-lead ECGs.
Additional Findings Observed in Clinical Trials
Adverse Reactions in Long-Term, Double-Blind, Placebo-Controlled Trials
- The adverse reactions reported in a 26-week, double-blind trial comparing oral ABILIFY and placebo in patients with schizophrenia were generally consistent with those reported in the short-term, placebo-controlled trials, except for a higher incidence of tremor [8% (12/153) for ABILIFY vs. 2% (3/153) for placebo]. In this study, the majority of the cases of tremor were of mild intensity (8/12 mild and 4/12 moderate), occurred early in therapy (9/12 ≤49 days), and were of limited duration (7/12 ≤10 days). Tremor infrequently led to discontinuation (<1%) of ABILIFY. In addition, in a long-term (52-week), active-controlled study, the incidence of tremor was 5% (40/859) for ABILIFY. A similar profile was observed in a long-term monotherapy study and a long-term adjunctive study with lithium and valproate in bipolar disorder.
Other Adverse Reactions Observed During the Premarketing Evaluation of Aripiprazole
- Following is a list of MedDRA terms that reflect adverse reactions as defined in ADVERSE REACTIONS (6.1) reported by patients treated with oral aripiprazole at multiple doses ≥2 mg/day during any phase of a trial within the database of 13,543 adult patients. All events assessed as possible adverse drug reactions have been included with the exception of more commonly occurring events. In addition, medically/clinically meaningful adverse reactions, particularly those that are likely to be useful to the prescriber or that have pharmacologic plausibility, have been included. Events already listed in other parts of ADVERSE REACTIONS (6), or those considered in WARNINGS AND PRECAUTIONS (5) or OVERDOSAGE (10) have been excluded. Although the reactions reported occurred during treatment with aripiprazole, they were not necessarily caused by it.
- Events are further categorized by MedDRA system organ class and listed in order of decreasing frequency according to the following definitions: those occurring in at least 1/100 patients (only those not already listed in the tabulated results from placebo-controlled trials appear in this listing); those occurring in 1/100 to 1/1000 patients; and those occurring in fewer than 1/1000 patients.
Adults - Oral Administration
- Blood and Lymphatic System Disorders:
≥1/1000 patients and <1/100 patients - leukopenia, neutropenia, thrombocytopenia
- Cardiac Disorders:
≥1/1000 patients and <1/100 patients - bradycardia, palpitations, cardiopulmonary failure, myocardial infarction, cardio-respiratory arrest, atrioventricular block, extrasystoles, sinus tachycardia, atrial fibrillation, angina pectoris, myocardial ischemia; <1/1000 patients - atrial flutter, supraventricular tachycardia, ventricular tachycardia
- Eye Disorders:
≥1/1000 patients and <1/100 patients - photophobia, diplopia, eyelid edema, photopsia
- Gastrointestinal Disorders:
≥1/1000 patients and <1/100 patients - gastroesophageal reflux disease, swollen tongue, esophagitis; <1/1000 patients - pancreatitis
- General Disorders and Administration Site Conditions:
≥1/100 patients - asthenia, peripheral edema, chest pain; ≥1/1000 patients and <1/100 patients - face edema, angioedema; <1/1000 patients - hypothermia Hepatobiliary Disorders:
<1/1000 patients - hepatitis, jaundice
- Immune System Disorders:
≥1/1000 patients and <1/100 patients - hypersensitivity Injury, Poisoning, and Procedural Complications:
≥1/100 patients - fall; ≥1/1000 patients and <1/100 patients - self mutilation; <1/1000 patients - heat stroke
- Investigations:
≥1/100 patients - weight decreased, creatine phosphokinase increased; ≥1/1000 patients and <1/100 patients - hepatic enzyme increased, blood glucose increased, blood prolactin increased, blood urea increased, electrocardiogram QT prolonged, blood creatinine increased, blood bilirubin increased; <1/1000 patients - blood lactate dehydrogenase increased, glycosylated hemoglobin increased, gamma-glutamyl transferase increased
- Metabolism and Nutrition Disorders:
≥1/1000 patients and <1/100 patients - hyperlipidemia, anorexia, diabetes mellitus (including blood insulin increased, carbohydrate tolerance decreased, diabetes mellitus non-insulin-dependent, glucose tolerance impaired, glycosuria, glucose urine, glucose urine present), hyperglycemia, hypokalemia, hyponatremia, hypoglycemia, polydipsia; <1/1000 patients - diabetic ketoacidosis
- Musculoskeletal and Connective Tissue Disorders:
≥1/1000 patients and <1/100 patients - muscle rigidity, muscular weakness, muscle tightness, mobility decreased; <1/1000 patients - rhabdomyolysis
- Nervous System Disorders:
≥1/100 patients - coordination abnormal; ≥1/1000 patients and <1/100 patients - speech disorder, parkinsonism, memory impairment, cogwheel rigidity, cerebrovascular accident, hypokinesia, tardive dyskinesia, hypotonia, myoclonus, hypertonia, akinesia, bradykinesia; <1/1000 patients - Grand Mal convulsion, choreoathetosis
- Psychiatric Disorders:
≥1/100 patients - suicidal ideation; ≥1/1000 patients and <1/100 patients - aggression, loss of libido, suicide attempt, hostility, libido increased, anger, anorgasmia, delirium, intentional self injury, completed suicide, tic, homicidal ideation; <1/1000 patients - catatonia, sleep walking
- Renal and Urinary Disorders:
≥1/1000 patients and <1/100 patients - urinary retention, polyuria, nocturia Reproductive System and Breast Disorders:
≥1/1000 patients and <1/100 patients - menstruation irregular, erectile dysfunction, amenorrhea, breast pain; <1/1000 patients - gynaecomastia, priapism
- Respiratory, Thoracic, and Mediastinal Disorders:
≥1/100 patients - nasal congestion, dyspnea, pneumonia aspiration
- Skin and Subcutaneous Tissue Disorders:
≥1/100 patients - rash (including erythematous, exfoliative, generalized, macular, maculopapular, papular rash; acneiform, allergic, contact, exfoliative, seborrheic dermatitis, neurodermatitis, and drug eruption), hyperhydrosis; ≥1/1000 patients and <1/100 patients - pruritus, photosensitivity reaction, alopecia, urticaria
- Vascular Disorders:
≥1/100 patients - hypertension; ≥1/1000 patients and <1/100 patients - hypotension
Pediatric Patients - Oral Administration
Most adverse events observed in the pooled database of 920 pediatric patients, aged 6 to 17 years, were also observed in the adult population. Additional adverse reactions observed in the pediatric population are listed below.
- Gastrointestinal Disorders:
≥1/1000 patients and <1/100 patients - tongue dry, tongue spasm
- Investigations:
≥1/100 patients - blood insulin increased
- Nervous System Disorders:
≥1/1000 patients and <1/100 patients - sleep talking
- Skin and Subcutaneous Tissue Disorders:
≥1/1000 patients and <1/100 patients - hirsutism
Adults - Intramuscular Injection
- Most adverse reactions observed in the pooled database of 749 adult patients treated with aripiprazole injection, were also observed in the adult population treated with oral aripiprazole. Additional adverse reactions observed in the aripiprazole injection population are listed below.
- General Disorders and Administration Site Conditions:
≥1/100 patients - injection site reaction; ≥1/1000 patients and <1/100 patients - venipuncture site bruise.
- (list/description of adverse reactions)
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of ABILIFY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to establish a causal relationship to drug exposure: rare occurrences of allergic reaction (anaphylactic reaction, angioedema, laryngospasm, pruritus/urticaria, or oropharyngeal spasm), and blood glucose fluctuation.
Drug Interactions
- Given the primary CNS effects of aripiprazole, caution should be used when ABILIFY is taken in combination with other centrally-acting drugs or alcohol.
- Due to its alpha adrenergic antagonism, aripiprazole has the potential to enhance the effect of certain antihypertensive agents.
Potential for Other Drugs to Affect ABILIFY
- Aripiprazole is not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2E1 enzymes. Aripiprazole also does not undergo direct glucuronidation. This suggests that an interaction of aripiprazole with inhibitors or inducers of these enzymes, or other factors, like smoking, is unlikely.
- Both CYP3A4 and CYP2D6 are responsible for aripiprazole metabolism. Agents that induce CYP3A4 (eg, carbamazepine) could cause an increase in aripiprazole clearance and lower blood levels. Inhibitors of CYP3A4 (eg, ketoconazole) or CYP2D6 (eg, quinidine, fluoxetine, or paroxetine) can inhibit aripiprazole elimination and cause increased blood level.
Ketoconazole and Other CYP3A4 Inhibitors
- Coadministration of ketoconazole (200 mg/day for 14 days) with a 15 mg single dose of aripiprazole increased the AUC of aripiprazole and its active metabolite by 63% and 77%, respectively. The effect of a higher ketoconazole dose (400 mg/day) has not been studied. When ketoconazole is given concomitantly with aripiprazole, the aripiprazole dose should be reduced to one-half of its normal dose.
- Other strong inhibitors of CYP3A4 (itraconazole) would be expected to have similar effects and need similar dose reductions; moderate inhibitors (erythromycin, grapefruit juice) have not been studied. When the CYP3A4 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should be increased.
Quinidine and Other CYP2D6 Inhibitors
- Coadministration of a 10 mg single dose of aripiprazole with quinidine (166 mg/day for 13 days), a potent inhibitor of CYP2D6, increased the AUC of aripiprazole by 112% but decreased the AUC of its active metabolite, dehydro-aripiprazole, by 35%. Aripiprazole dose should be reduced to one-half of its normal dose when quinidine is given concomitantly with aripiprazole.
- Other significant inhibitors of CYP2D6, such as fluoxetine or paroxetine, would be expected to have similar effects and should lead to similar dose reductions. When the CYP2D6 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should be increased. When adjunctive ABILIFY is administered to patients with major depressive disorder, ABILIFY should be administered without dosage adjustment.
Carbamazepine and Other CYP3A4 Inducers
- Coadministration of carbamazepine (200 mg twice daily), a potent CYP3A4 inducer, with aripiprazole (30 mg/day) resulted in an approximate 70% decrease in Cmax and AUC values of both aripiprazole and its active metabolite, dehydro-aripiprazole. When carbamazepine is added to aripiprazole therapy, aripiprazole dose should be doubled. Additional dose increases should be based on clinical evaluation. When carbamazepine is withdrawn from the combination therapy, the aripiprazole dose should be reduced
Potential for ABILIFY to Affect Other Drugs
- Aripiprazole is unlikely to cause clinically important pharmacokinetic interactions with drugs metabolized by cytochrome P450 enzymes. In in vivo studies, 10 mg/day to 30 mg/day doses of aripiprazole had no significant effect on metabolism by CYP2D6 (dextromethorphan), CYP2C9 (warfarin), CYP2C19 (omeprazole, warfarin), and CYP3A4 (dextromethorphan) substrates. Additionally, aripiprazole and dehydro-aripiprazole did not show potential for altering CYP1A2-mediated metabolism in vitro.
- No effect of aripiprazole was seen on the pharmacokinetics of lithium or valproate.
Drugs Having No Clinically Important Interactions with ABILIFY
Famotidine
- Coadministration of aripiprazole (given in a single dose of 15 mg) with a 40 mg single dose of the H2 antagonist famotidine, a potent gastric acid blocker, decreased the solubility of aripiprazole and, hence, its rate of absorption, reducing by 37% and 21% the Cmax of aripiprazole and dehydro-aripiprazole, respectively, and by 13% and 15%, respectively, the extent of absorption (AUC). No dosage adjustment of aripiprazole is required when administered concomitantly with famotidine.
Valproate
- When valproate (500 mg/day-1500 mg/day) and aripiprazole (30 mg/day) were coadministered, at steady-state the Cmax and AUC of aripiprazole were decreased by 25%. No dosage adjustment of aripiprazole is required when administered concomitantly with valproate.
- When aripiprazole (30 mg/day) and valproate (1000 mg/day) were coadministered, at steady-state there were no clinically significant changes in the Cmax or AUC of valproate. No dosage adjustment of valproate is required when administered concomitantly with aripiprazole.
Lithium
- A pharmacokinetic interaction of aripiprazole with lithium is unlikely because lithium is not bound to plasma proteins, is not metabolized, and is almost entirely excreted unchanged in urine. Coadministration of therapeutic doses of lithium (1200 mg/day-1800 mg/day) for 21 days with aripiprazole (30 mg/day) did not result in clinically significant changes in the pharmacokinetics of aripiprazole or its active metabolite, dehydro-aripiprazole (Cmax and AUC increased by less than 20%). No dosage adjustment of aripiprazole is required when administered concomitantly with lithium.
- Coadministration of aripiprazole (30 mg/day) with lithium (900 mg/day) did not result in clinically significant changes in the pharmacokinetics of lithium. No dosage adjustment of lithium is required when administered concomitantly with aripiprazole.
Lamotrigine
- Coadministration of 10 mg/day to 30 mg/day oral doses of aripiprazole for 14 days to patients with bipolar I disorder had no effect on the steady-state pharmacokinetics of 100 mg/day to 400 mg/day lamotrigine, a UDP-glucuronosyltransferase 1A4 substrate. No dosage adjustment of lamotrigine is required when aripiprazole is added to lamotrigine.
Dextromethorphan
- Aripiprazole at doses of 10 mg/day to 30 mg/day for 14 days had no effect on dextromethorphan's O-dealkylation to its major metabolite, dextrorphan, a pathway dependent on CYP2D6 activity. Aripiprazole also had no effect on dextromethorphan's N-demethylation to its metabolite 3-methoxymorphinan, a pathway dependent on CYP3A4 activity. No dosage adjustment of dextromethorphan is required when administered concomitantly with aripiprazole.
Warfarin
- Aripiprazole 10 mg/day for 14 days had no effect on the pharmacokinetics of R-warfarin and S-warfarin or on the pharmacodynamic end point of International Normalized Ratio, indicating the lack of a clinically relevant effect of aripiprazole on CYP2C9 and CYP2C19 metabolism or the binding of highly protein-bound warfarin. No dosage adjustment of warfarin is required when administered concomitantly with aripiprazole.
Omeprazole
- Aripiprazole 10 mg/day for 15 days had no effect on the pharmacokinetics of a single 20 mg dose of omeprazole, a CYP2C19 substrate, in healthy subjects. No dosage adjustment of omeprazole is required when administered concomitantly with aripiprazole.
Lorazepam
- Coadministration of lorazepam injection (2 mg) and aripiprazole injection (15 mg) to healthy subjects (n=40: 35 males and 5 females; ages 19-45 years old) did not result in clinically important changes in the pharmacokinetics of either drug. No dosage adjustment of aripiprazole is required when administered concomitantly with lorazepam. However, the intensity of sedation was greater with the combination as compared to that observed with aripiprazole alone and the orthostatic hypotension observed was greater with the combination as compared to that observed with lorazepam alone.
Escitalopram
- Coadministration of 10 mg/day oral doses of aripiprazole for 14 days to healthy subjects had no effect on the steady-state pharmacokinetics of 10 mg/day escitalopram, a substrate of CYP2C19 and CYP3A4. No dosage adjustment of escitalopram is required when aripiprazole is added to escitalopram.
Venlafaxine
- Coadministration of 10 mg/day to 20 mg/day oral doses of aripiprazole for 14 days to healthy subjects had no effect on the steady-state pharmacokinetics of venlafaxine and O-desmethylvenlafaxine following 75 mg/day venlafaxine XR, a CYP2D6 substrate. No dosage adjustment of venlafaxine is required when aripiprazole is added to venlafaxine.
Fluoxetine, Paroxetine, and Sertraline
- A population pharmacokinetic analysis in patients with major depressive disorder showed no substantial change in plasma concentrations of fluoxetine (20 mg/day or 40 mg/day), paroxetine CR (37.5 mg/day or 50 mg/day), or sertraline (100 mg/day or 150 mg/day) dosed to steady-state. The steady-state plasma concentrations of fluoxetine and norfluoxetine increased by about 18% and 36%, respectively, and concentrations of paroxetine decreased by about 27%. The steady-state plasma concentrations of sertraline and desmethylsertraline were not substantially changed when these antidepressant therapies were coadministered with aripiprazole. Aripiprazole dosing was 2 mg/day to 15 mg/day (when given with fluoxetine or paroxetine) or 2 mg/day to 20 mg/day (when given with sertraline).
Use in Specific Populations
Pregnancy
- In general, no dosage adjustment for ABILIFY is required on the basis of a patient’s age, gender, race, smoking status, hepatic function, or renal function.
Pregnancy
Teratogenic Effects
- Pregnancy Category C: In animal studies, aripiprazole demonstrated developmental toxicity, including possible teratogenic effects in rats and rabbits.
- Pregnant rats were treated with oral doses of 3 mg/kg/day, 10 mg/kg/day, and 30 mg/kg/day (1 times, 3 times, and 10 times the maximum recommended human dose [MRHD] on a mg/m2 basis) of aripiprazole during the period of organogenesis. Gestation was slightly prolonged at 30 mg/kg. Treatment caused a slight delay in fetal development, as evidenced by decreased fetal weight (30 mg/kg), undescended testes (30 mg/kg), and delayed skeletal ossification (10 mg/kg and 30 mg/kg). There were no adverse effects on embryofetal or pup survival. Delivered offspring had decreased body weights (10 mg/kg and 30 mg/kg), and increased incidences of hepatodiaphragmatic nodules and diaphragmatic hernia at 30 mg/kg (the other dose groups were not examined for these findings). A low incidence of diaphragmatic hernia was also seen in the fetuses exposed to 30 mg/kg. Postnatally, delayed vaginal opening was seen at 10 mg/kg and 30 mg/kg and impaired reproductive performance (decreased fertility rate, corpora lutea, implants, live fetuses, and increased post-implantation loss, likely mediated through effects on female offspring) was seen at 30 mg/kg. Some maternal toxicity was seen at 30 mg/kg; however, there was no evidence to suggest that these developmental effects were secondary to maternal toxicity.
- In pregnant rats receiving aripiprazole injection intravenously (3 mg/kg/day, 9 mg/kg/day, and 27 mg/kg/day) during the period of organogenesis, decreased fetal weight and delayed skeletal ossification were seen at the highest dose, which also caused some maternal toxicity.
- Pregnant rabbits were treated with oral doses of 10 mg/kg/day, 30 mg/kg/day, and 100 mg/kg/day (2 times, 3 times, and 11 times human exposure at MRHD based on AUC and 6 times, 19 times, and 65 times the MRHD based on mg/m2) of aripiprazole during the period of organogenesis. Decreased maternal food consumption and increased abortions were seen at 100 mg/kg. Treatment caused increased fetal mortality (100 mg/kg), decreased fetal weight (30 mg/kg and 100 mg/kg), increased incidence of a skeletal abnormality (fused sternebrae at 30 mg/kg and 100 mg/kg), and minor skeletal variations (100 mg/kg).
- In pregnant rabbits receiving aripiprazole injection intravenously (3 mg/kg/day, 10 mg/kg/day, and 30 mg/kg/day) during the period of organogenesis, the highest dose, which caused pronounced maternal toxicity, resulted in decreased fetal weight, increased fetal abnormalities (primarily skeletal), and decreased fetal skeletal ossification. The fetal no-effect dose was 10 mg/kg, which produced 5 times the human exposure at the MRHD based on AUC and is 6 times the MRHD based on mg/m2.
- In a study in which rats were treated with oral doses of 3 mg/kg/day, 10 mg/kg/day, and 30 mg/kg/day (1 times, 3 times, and 10 times the MRHD on a mg/m2 basis) of aripiprazole perinatally and postnatally (from day 17 of gestation through day 21 postpartum), slight maternal toxicity and slightly prolonged gestation were seen at 30 mg/kg. An increase in stillbirths and decreases in pup weight (persisting into adulthood) and survival were seen at this dose.
- In rats receiving aripiprazole injection intravenously (3 mg/kg/day, 8 mg/kg/day, and 20 mg/kg/day) from day 6 of gestation through day 20 postpartum, an increase in stillbirths was seen at 8 mg/kg and 20 mg/kg, and decreases in early postnatal pup weights and survival were seen at 20 mg/kg. These doses produced some maternal toxicity. There were no effects on postnatal behavioral and reproductive development.
Non-teratogenic Effects
- There are no adequate and well-controlled studies in pregnant women. It is not known whether aripiprazole can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates
Pregnancy Category (AUS):
(Description)
Labor and Delivery
- The effect of aripiprazole on labor and delivery in humans is unknown.
Nursing Mothers
- Aripiprazole is excreted in human breast milk. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients with major depressive disorder or agitation associated with schizophrenia or bipolar mania have not been established.
- Safety and effectiveness in pediatric patients with schizophrenia were established in a 6-week, placebo-controlled clinical trial in 202 pediatric patients aged 13 to 17 years . Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
- Safety and effectiveness in pediatric patients with bipolar mania were established in a 4-week, placebo-controlled clinical trial in 197 pediatric patients aged 10 to 17 years . Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
- The efficacy of adjunctive ABILIFY with concomitant lithium or valproate in the treatment of manic or mixed episodes in pediatric patients has not been systematically evaluated. However, such efficacy and lack of pharmacokinetic interaction between aripiprazole and lithium or valproate can be extrapolated from adult data, along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
- Safety and effectiveness in pediatric patients demonstrating irritability associated with autistic disorder were established in two 8-week, placebo-controlled clinical trials in 212 pediatric patients aged 6 to 17 years . A maintenance trial was conducted in pediatric patients (6 to 17 years of age) with irritability associated with autistic disorder. The first phase of this trial was an open-label, flexibly dosed (aripiprazole 2 to 15 mg/day) phase in which patients were stabilized (defined as > 25% improvement on the ABC-I subscale, and a CGI-I rating of “much improved” or “very much improved”) on ABILIFY for 12 consecutive weeks. Overall, 85 patients were stabilized and entered the second, 16-week, double-blind phase where they were randomized to either continue ABILIFY treatment or switch to placebo. In this trial, the efficacy of ABILIFY for the maintenance treatment of irritability associated with autistic disorder was not established.
- The pharmacokinetics of aripiprazole and dehydro-aripiprazole in pediatric patients, 10 to 17 years of age, were similar to those in adults after correcting for the differences in body weight
Geriatic Use
- In formal single-dose pharmacokinetic studies (with aripiprazole given in a single dose of 15 mg), aripiprazole clearance was 20% lower in elderly (≥65 years) subjects compared to younger adult subjects (18 to 64 years). There was no detectable age effect, however, in the population pharmacokinetic analysis in schizophrenia patients. Also, the pharmacokinetics of aripiprazole after multiple doses in elderly patients appeared similar to that observed in young, healthy subjects. No dosage adjustment is recommended for elderly patients [see also BOXED WARNING and WARNINGS AND PRECAUTIONS].
- Of the 13,543 patients treated with oral aripiprazole in clinical trials, 1073 (8%) were ≥65 years old and 799 (6%) were ≥75 years old. The majority (81%) of the 1073 patients were diagnosed with Dementia of the Alzheimer's type.
- Placebo-controlled studies of oral aripiprazole in schizophrenia, bipolar mania, or major depressive disorder did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
- Of the 749 patients treated with aripiprazole injection in clinical trials, 99 (13%) were ≥65 years old and 78 (10%) were ≥75 years old. Placebo-controlled studies of aripiprazole injection in patients with agitation associated with schizophrenia or bipolar mania did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
- Studies of elderly patients with psychosis associated with Alzheimer's disease have suggested that there may be a different tolerability profile in this population compared to younger patients with schizophrenia [see also BOXED WARNING and WARNINGS AND PRECAUTIONS]. The safety and efficacy of ABILIFY in the treatment of patients with psychosis associated with Alzheimer's disease has not been established. If the prescriber elects to treat such patients with ABILIFY, vigilance should be exercised.
Gender
- Cmax and AUC of aripiprazole and its active metabolite, dehydro-aripiprazole, are 30% to 40% higher in women than in men, and correspondingly, the apparent oral clearance of aripiprazole is lower in women. These differences, however, are largely explained by differences in body weight (25%) between men and women. No dosage adjustment is recommended based on gender.
Race
- Although no specific pharmacokinetic study was conducted to investigate the effects of race on the disposition of aripiprazole, population pharmacokinetic evaluation revealed no evidence of clinically significant race-related differences in the pharmacokinetics of aripiprazole. No dosage adjustment is recommended based on race.
Renal Impairment
- In patients with severe renal impairment (creatinine clearance <30 mL/min), Cmax of aripiprazole (given in a single dose of 15 mg) and dehydro-aripiprazole increased by 36% and 53%, respectively, but AUC was 15% lower for aripiprazole and 7% higher for dehydro-aripiprazole. Renal excretion of both unchanged aripiprazole and dehydro-aripiprazole is less than 1% of the dose. No dosage adjustment is required in subjects with renal impairment.
Hepatic Impairment
- In a single-dose study (15 mg of aripiprazole) in subjects with varying degrees of liver cirrhosis (Child-Pugh Classes A, B, and C), the AUC of aripiprazole, compared to healthy subjects, increased 31% in mild HI, increased 8% in moderate HI, and decreased 20% in severe HI. None of these differences would require dose adjustment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aripiprazole (intramuscular) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aripiprazole (intramuscular) in patients who are immunocompromised.
Others
- Smoking
- Based on studies utilizing human liver enzymes in vitro, aripiprazole is not a substrate for CYP1A2 and also does not undergo direct glucuronidation. Smoking should, therefore, not have an effect on the pharmacokinetics of aripiprazole. Consistent with these in vitro results, population pharmacokinetic evaluation did not reveal any significant pharmacokinetic differences between smokers and nonsmokers. No dosage adjustment is recommended based on smoking status.
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding IV Compatibility of Aripiprazole in the drug label.
Overdosage
OVERDOSAGE
- MedDRA terminology has been used to classify the adverse reactions.
Human Experience
- In clinical trials and in postmarketing experience, adverse reactions of deliberate or accidental overdosage with oral aripiprazole have been reported worldwide. These include overdoses with oral aripiprazole alone and in combination with other substances. No fatality was reported with aripiprazole alone. The largest known dose with a known outcome involved acute ingestion of 1260 mg of oral aripiprazole (42 times the maximum recommended daily dose) by a patient who fully recovered. Deliberate or accidental overdosage was also reported in children (age 12 and younger) involving oral aripiprazole ingestions up to 195 mg with no fatalities.
- Common adverse reactions (reported in at least 5% of all overdose cases) reported with oral aripiprazole overdosage (alone or in combination with other substances) include vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.
Management of Overdosage
- No specific information is available on the treatment of overdose with aripiprazole. An electrocardiogram should be obtained in case of overdosage and if QT interval prolongation is present, cardiac monitoring should be instituted. Otherwise, management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers.
- Charcoal: In the event of an overdose of ABILIFY, an early charcoal administration may be useful in partially preventing the absorption of aripiprazole. Administration of 50 g of activated charcoal, one hour after a single 15 mg oral dose of aripiprazole, decreased the mean AUC and Cmax of aripiprazole by 50%.
- Hemodialysis: Although there is no information on the effect of hemodialysis in treating an overdose with aripiprazole, hemodialysis is unlikely to be useful in overdose management since aripiprazole is highly bound to plasma proteins.
)
Pharmacology
Mechanism of Action
(Description)
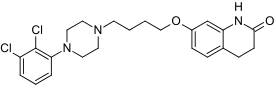
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Aripiprazole (intramuscular) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Aripiprazole (intramuscular) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Aripiprazole (intramuscular) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Aripiprazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Aripiprazole (intramuscular) Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Bellino S, Paradiso E, Bogetto F (2008). "Efficacy and tolerability of aripiprazole augmentation in sertraline-resistant patients with borderline personality disorder". Psychiatry Res. 161 (2): 206–12. doi:10.1016/j.psychres.2007.07.006. PMID 18848360.
- ↑ 2.0 2.1 2.2 2.3 2.4
- ↑ 3.0 3.1 3.2 3.3 3.4 "ABILIFY (aripiprazole) tablet ABILIFY (aripiprazole) solution ABILIFY DISCMELT (aripiprazole) tablet, orally disintegrating ABILIFY (aripiprazole) injection, solution [Otsuka America Pharmaceutical, Inc.]". DailyMed. Otsuka America Pharmaceutical, Inc. April 2013. Retrieved 22 October 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Abilify Tablets, Orodispersible Tablets, Oral Solution - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Otsuka Pharmaceuticals (UK) Ltd. 20 September 2013. Retrieved 22 October 2013.
- ↑ 5.0 5.1 5.2 5.3 5.4 "ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS" (PDF). European Medicines Agency. Otsuka Pharmaceutical Europe Ltd. Retrieved 22 October 2013.
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480008.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;008;10 |Pill Dosage=10 mg |Pill Color=Pink|+sep=; |Pill Shape= |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480008
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480006.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;006;2 |Pill Dosage=2 mg |Pill Color=Green|+sep=; |Pill Shape= |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480006
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480007.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;007;5 |Pill Dosage=5 mg |Pill Color=Blue|+sep=; |Pill Shape= |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480007
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480009.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;009;15 |Pill Dosage=15 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480009
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480010.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;010;20 |Pill Dosage=20 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480010
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480011.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;011;30 |Pill Dosage=30 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480011
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_01.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_02.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_03.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_04.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_05.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_06.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_07.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_08.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_label_09.jpg
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_01.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_02.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_03.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_04.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_05.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_06.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_07.png
}}
{{#subobject:
|Label Page=Aripiprazole (intramuscular) |Label Name=Aripiprazole_panel_08.png
}}