Apixaban: Difference between revisions
No edit summary |
m (Protected "Apixaban": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
(No difference)
| |
Revision as of 18:52, 27 September 2011
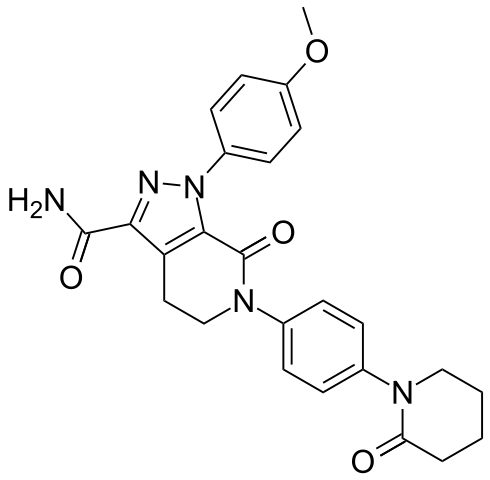 | |
| Clinical data | |
|---|---|
| Routes of administration | oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C25H25N5O4 |
| Molar mass | 459.497 |
|
WikiDoc Resources for Apixaban |
|
Articles |
|---|
|
Most recent articles on Apixaban |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Apixaban at Clinical Trials.gov Clinical Trials on Apixaban at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Apixaban
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Apixaban Risk calculators and risk factors for Apixaban
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Apixaban |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Apixaban (manufacturer's designation BMS-562247-01) is a compound being investigated as an anticoagulant. It acts by inhibiting coagulation factor Xa. It is presently undergoing phase III trials in the prevention of venous thromboembolism, together with a number of related competing compounds, such as rivaroxaban.[1] It is being marketed in a joint venture by Pfizer and Bristol-Myers-Squibb.[2]
Acute Coronary Syndrome (ACS) Studies
APPRAISE-1 Trial
Results of the Dose Ranging Study to Evaluate Safety and Efficacy of Apixaban in Patients with a Recent Acute Coronary Syndrome (APPRAISE-1) demonstrate that administration of the factor Xa inhibitor Apixaban on top of current antiplatelet therapy for 6 months post-acute coronary syndrome (ACS) leads to increased bleeding in a dose-dependent fashion and reduced ischemic events. The data were presented by Dr. John H. Alexander and Dr. Lars Wallentin at the European Society of Cardiology Congress 2008 Hot Line III session.
The goal of this trial was 1) to evaluate the effect on bleeding of 4 doses of Apixaban vs. placebo given over 26 weeks in patients with a recent ACS receiving current evidence based care (Aspirin ≤165 mg/d, Clopidogrel per MD discretion) and 2) to determine the optimal dose of Apixaban for further investigation. Major bleeding was defined as bleeding with a fall in hemoglobin of ≥2 g/dl, or bleeding with transfusion of ≥2 units of PRBC or whole blood, or bleeding that occurs in a critical location (i.e. brain or spine), or bleeding that causes death.
The APPRAISE-1 trial was a phase 2, randomized, double-blind, placebo-controlled, parallel arm study that enrolled 1,715 patients from Europe (n=1,305) and North America (n=410). Phase A of the study randomized 547 patients to 10 mg Apixaban daily (Apixaban 10 mg QD, n=184), 2.5 mg Apixaban twice daily (Apixaban 2.5 mg BID, n=179), and placebo (n=184). In phase B, 1168 patients were randomized to Apixaban 10 mg QD (n=134), Apixaban 2.5 mg BID (n=138), placebo (n=427), Apixaban 10 mg BID (n=248), and Apixaban 20 mg QD (n=221). All patients received the study drug for 6 months. The latter two groups (10 mg BID and 20 mg QD, n=469) were discontinued prematurely from the study due to excess bleeding.
The inclusion criteria selected patients age 18-90 years with recent ACS (≤7 days) and at least one additional risk factor such as age ≥65 years, diabetes mellitus, or prior MI within the past 12 months. The primary safety endpoint was International Society of Thrombosis and Haemostasis (ISTH) major or clinically relevant non-major (CRNM) bleeding. The secondary efficacy endpoint included cardiovascular (CV) death, MI, severe recurrent ischemia, or ischemic stroke. The duration of follow-up was a mean of 6 months.
Compared to placebo, Apixaban treatment was associated with increased bleeding among patients with a recent ACS according to the ISTH major or CRNM and Thrombolysis in Myocardial Infarction (TIMI) bleeding definitions. According to the ISTH major or CRNM definition, bleeding occurred in 7.9% of patients receiving Apixaban 10 mg QD (n=315), 5.7% in the Apixaban 2.5 mg BID arm (n=315) and 3.0% in the placebo group (n=599). HR: 2.45, 95% CI: 1.31 to 4.61, p = 0.005 for Apixaban 10 mg QD vs. placebo and HR: 1.78, 95% CI: 0.91 to 3.48, p = 0.09 for Apixaban 2.5 mg BID vs. placebo. 1.0%, 0.0%, 0.3% of patients being administered Apixaban 10 mg QD (n=315), Apixaban 2.5 mg BID (n=315) and placebo (n=599), respectively, experienced major bleeding by the TIMI definition.
When bleeding was stratified by Clopidogrel status, the study drug was still associated with increased bleeding. Patients taking Clopidogrel: 9.1% bleeding for Apixaban 10 mg QD (n=241), 7.0% for Apixaban 2.5 mg BID (n=230), 3.1% for placebo (n=453); No Clopidogrel: 4.1%, 2.4%, 2.7% bleeding for Apixaban 10 mg QD (n=74), Apixaban 2.5 mg BID (n=85) and placebo (n=146) respectively.
The secondary endpoints of CV death, MI, severe recurrent ischemia, or ischemic stroke were most prevalent in the placebo group (6.0%, 7.6%, and 8.7% in the Apixaban 10 mg QD n=318, Apixaban 2.5 mg BID n=317 and placebo groups n=611, respectively, HR 0.61, 95% CI 0.35 to 1.04, p = 0.07 for Apixaban 10 mg QD vs. placebo and HR 0.73, 95% CI 0.44 to 1.19, p = 0.21 for Apixaban 2.5 mg BID vs. placebo) while CV death alone occurred most frequently in the Apixaban 2.5 mg BID group (1.3% Apixaban 10 mg QD n = 318, 3.5% Apixaban 2.5 mg BID n = 317, 1.8% placebo n = 611).
When secondary endpoints were stratified by Clopidogrel status, placebo was associated with the most ischemic events. Patients taking Clopidogrel: 4.9% for Apixaban 10 mg QD (n=243), 5.6% for Apixaban 2.5 mg BID (n=232), 6.5 % for placebo (n=462); No Clopidogrel: 9.3%, 12.9%, 15.4% for Apixaban 10 mg QD (n=75), Apixaban 2.5 mg BID (n=85) and placebo (n=149) respectively.
APPRAISE-1 represents the first trial to combine anticoagulation with a direct factor Xa inhibitor in the treatment of patients with a recent ACS already receiving antiplatelet therapy. Overall, the results suggest that administration of Apixaban on top of current antiplatelet therapy for 6 months post-ACS leads to increased bleeding in a dose-dependent fashion and reduced ischemic events. Similar outcomes were found among patients taking Aspirin or Aspirin + Clopidogrel.
APPRAISE-1 was supported by Bristol-Myers Squibb.
References
- John H. Alexander, Lars Wallentin, APPRAISE-1 Committees and Investigators. Safety of the factor Xa inhibitor, Apixaban, in combination with antiplatelet therapy after acute coronary syndromes: Results from the APPRAISE-1 dose guiding trial. As presented at ESC 2008.
Venous Thromboembolism (VTE) Studies
A 2007 trial showed that apixaban was equivalent to enoxaparin/open-label heparin in preventing thrombosis is patients who had undergone a knee replacement.[3]
References
- ↑ Turpie AG (2007). "Oral, direct factor Xa inhibitors in development for the prevention and treatment of thromboembolic diseases". Arterioscler. Thromb. Vasc. Biol. 27 (6): 1238–47. doi:10.1161/ATVBAHA.107.139402. PMID 17379841.
- ↑ "Bristol-Myers Squibb News Release 26 April 2007". Retrieved 2007-09-15.
- ↑ Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D (2007). "The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement". J. Thromb. Haemost. 5 (12): 2368–75. doi:10.1111/j.1538-7836.2007.02764.x. PMID 17868430.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Anticoagulants
- Pyrazoles