Angiostrongylus cantonensis: Difference between revisions
Gerald Chi- (talk | contribs) |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 21: | Line 21: | ||
==Overview== | ==Overview== | ||
'''''Angiostrongylus cantonensis''''' is a [[Tapeworm infection|parasitic]] [[nematode]] (roundworm) that causes [[Angiostrongyliasis]], the most common cause of [[eosinophilic meningitis]] in [[Southeast Asia]] and the [[Pacific Basin]]. | '''''Angiostrongylus cantonensis''''' is a [[Tapeworm infection|parasitic]] [[nematode]] (roundworm) that causes [[Angiostrongyliasis]], the most common cause of [[eosinophilic meningitis]] in [[Southeast Asia]] and the [[Pacific Basin]]. The nematode commonly resides in the pulmonary arteries of rats, giving it the nickname the '''rat lungworm'''. [[Snail]]s are the primary intermediate hosts, where larvae develop until they are infective. | ||
Humans are incidental hosts of this roundworm, and may become infected through ingestion of larvae in raw or undercooked snails or other vectors, or from contaminated water and vegetables. The larvae are then transported via the blood to the [[central nervous system]] (CNS), where they are the most common cause of [[eosinophilic meningitis]], a serious condition that can lead to death or permanent brain and nerve damage. | Humans are incidental hosts of this roundworm, and may become infected through ingestion of larvae in raw or undercooked snails or other vectors, or from contaminated water and vegetables. The larvae are then transported via the blood to the [[central nervous system]] (CNS), where they are the most common cause of [[eosinophilic meningitis]], a serious condition that can lead to death or permanent brain and nerve damage. Identified in 1964, Angiostrongyliasis is an infection of increasing public health importance as [[globalization]] contributes to the geographic spread of the disease. | ||
==Infectious agent== | ==Infectious agent== | ||
| Line 29: | Line 29: | ||
''Angiostrongylus cantonensis'' is a [[parasitic worm|helminth]] of the [[phylum]] [[nematode|Nematoda]], [[order (biology)|order]] [[Strongylida]], and [[superfamily (zoology)|superfamily]] [[Metastrongyloidea]]. Nematodes are roundworms characterized by a tough outer cuticle, unsegmented bodies, and a fully developed [[Human gastrointestinal tract|gastrointestinal tract]]. The order Strongylida includes hookworms and lungworms. Metastrongyloidea are characterized as long, slender, threadlike worms that reside in the lungs of the definitive host.<ref name=Cambridge>[http://www.path.cam.ac.uk/~schisto/helminth_taxonomy/taxonomy_nematoda.html "Helminth Taxonomy - Phylum Nematoda"], Schistosomiasis Research Group, accessed 26 February 2009.</ref> ''[[Angiostrongylus costaricensis]]'' is a closely related worm that causes intestinal Angiostrongyliasis in Central and South America. | ''Angiostrongylus cantonensis'' is a [[parasitic worm|helminth]] of the [[phylum]] [[nematode|Nematoda]], [[order (biology)|order]] [[Strongylida]], and [[superfamily (zoology)|superfamily]] [[Metastrongyloidea]]. Nematodes are roundworms characterized by a tough outer cuticle, unsegmented bodies, and a fully developed [[Human gastrointestinal tract|gastrointestinal tract]]. The order Strongylida includes hookworms and lungworms. Metastrongyloidea are characterized as long, slender, threadlike worms that reside in the lungs of the definitive host.<ref name=Cambridge>[http://www.path.cam.ac.uk/~schisto/helminth_taxonomy/taxonomy_nematoda.html "Helminth Taxonomy - Phylum Nematoda"], Schistosomiasis Research Group, accessed 26 February 2009.</ref> ''[[Angiostrongylus costaricensis]]'' is a closely related worm that causes intestinal Angiostrongyliasis in Central and South America. | ||
''A. cantonensis'' is a nematode roundworm with 3 outer protective collagen layers, and a simple stomal opening or mouth with no lips or buccal cavity leading to a fully developed gastrointestinal tract. | ''A. cantonensis'' is a nematode roundworm with 3 outer protective collagen layers, and a simple stomal opening or mouth with no lips or buccal cavity leading to a fully developed gastrointestinal tract. Males have a small copulatory bursa at the posterior. Females have a “[[barber pole]]” shape down the middle of the body, which is created by the twisting together of the intestine and uterine tubules. The worms are long and slender - males are 15.9-19 mm in length, and females are 21-25 mm in length.<ref name="Michigan">http://animaldiversity.ummz.umich.edu/site/accounts/information/Angiostrongylus_cantonensis.html, Accessed 2/26/09</ref> | ||
==Life cycle== | ==Life cycle== | ||
The adult form of ''A. cantonensis'' resides in the pulmonary arteries of rodents, where it reproduces. After the eggs hatch in the arteries, larvae migrate up the pharynx and are then swallowed again by the rodent and passed in the stool. These first stage larvae then penetrate or are swallowed by snail intermediate hosts, where they transform into second stage larvae and then into third stage infective larvae. Humans and rats acquire the infection when they ingest contaminated snails or paratenic (transport) hosts including prawns, crabs, and frogs, or raw vegetables containing material from these intermediate and paratenic hosts. After passing through the gastrointestinal tract, the worms enter circulation. | The adult form of ''A. cantonensis'' resides in the pulmonary arteries of rodents, where it reproduces. After the eggs hatch in the arteries, larvae migrate up the pharynx and are then swallowed again by the rodent and passed in the stool. These first stage larvae then penetrate or are swallowed by snail intermediate hosts, where they transform into second stage larvae and then into third stage infective larvae. Humans and rats acquire the infection when they ingest contaminated snails or paratenic (transport) hosts including prawns, crabs, and frogs, or raw vegetables containing material from these intermediate and paratenic hosts. After passing through the gastrointestinal tract, the worms enter circulation. In rats, the larvae then migrate to the meninges and develop for about a month before migrating to the pulmonary arteries, where they fully develop into adults. | ||
Humans are incidental hosts; the larvae cannot reproduce in humans and therefore humans do not contribute to the ''A. cantonensis'' life cycle. In humans, the circulating larvae migrate to the meninges, but do not move on to the lungs. Sometimes the larvae will develop into the adult form in the brain and CSF, but they quickly die, inciting the inflammatory reaction that causes symptoms of infection. | Humans are incidental hosts; the larvae cannot reproduce in humans and therefore humans do not contribute to the ''A. cantonensis'' life cycle. In humans, the circulating larvae migrate to the meninges, but do not move on to the lungs. Sometimes the larvae will develop into the adult form in the brain and CSF, but they quickly die, inciting the inflammatory reaction that causes symptoms of infection. | ||
<gallery widths=200px> | <gallery widths=200px> | ||
| Line 45: | Line 45: | ||
==Reservoirs== | ==Reservoirs== | ||
Rats are the definitive host and the main reservoir for ''A. cantonensis,'' though other small mammals may also become infected. While ''angiostrongylus'' can infect humans, humans do not act as reservoirs since the worm cannot reproduce in humans and therefore humans cannot contribute to their life cycle. | Rats are the definitive host and the main reservoir for ''A. cantonensis,'' though other small mammals may also become infected. While ''angiostrongylus'' can infect humans, humans do not act as reservoirs since the worm cannot reproduce in humans and therefore humans cannot contribute to their life cycle. | ||
==Vectors== | ==Vectors== | ||
''A. cantonensis'' has many vectors, with the most common being several species of snails, including the giant African land snail (''Achatina fulica'') in the Pacific islands and snails of the genus ''Pila'' in Thailand and Malaysia. The golden apple snail, ''A. canaliculatus'', is the most important vector in areas of China. | ''A. cantonensis'' has many vectors, with the most common being several species of snails, including the giant African land snail (''Achatina fulica'') in the Pacific islands and snails of the genus ''Pila'' in Thailand and Malaysia. The golden apple snail, ''A. canaliculatus'', is the most important vector in areas of China. Freshwater prawns, crabs, or other [[paratenic]], or transport, hosts can also act as vectors. | ||
==Hosts== | ==Hosts== | ||
Intermediate hosts of larvae of for ''Angiostrongylus cantonensis'' include: | Intermediate hosts of larvae of for ''Angiostrongylus cantonensis'' include: | ||
* land snails: ''[[Thelidomus aspera]]'' from Jamaica,<ref name="Lindo">Lindo J. F., Waugh C., Hall J., Cunningham-Myrie C., Ashley D., Eberhard M. L., Sullivan J. J., Bishop H. S., Robinson D. G., Holtz T. & Robinson R. D. (2002). "Enzootic ''Angiostrongylus cantonensis'' in Rats and Snails after an Outbreak of Human Eosinophilic Meningitis, Jamaica". ''[[Emerging Infectious Diseases]]'' '''8'''(3): 324-326. [http://www.cdc.gov/ncidod/eid/vol8no3/01-0316.htm HTM].</ref> ''[[Achatina fulica]]'', | * land snails: ''[[Thelidomus aspera]]'' from Jamaica,<ref name="Lindo">Lindo J. F., Waugh C., Hall J., Cunningham-Myrie C., Ashley D., Eberhard M. L., Sullivan J. J., Bishop H. S., Robinson D. G., Holtz T. & Robinson R. D. (2002). "Enzootic ''Angiostrongylus cantonensis'' in Rats and Snails after an Outbreak of Human Eosinophilic Meningitis, Jamaica". ''[[Emerging Infectious Diseases]]'' '''8'''(3): 324-326. [http://www.cdc.gov/ncidod/eid/vol8no3/01-0316.htm HTM].</ref> ''[[Achatina fulica]]'',<ref name="Lv2009"/><ref name="Asaro"/><ref name="airforcemedicine 2001"/> ''[[Satsuma mercatoria]]'',<ref name="Asaro"/><ref name="airforcemedicine 2001"/> ''[[Acusta despecta]]'',<ref name="Asaro"/><ref name="airforcemedicine 2001"/> ''[[Bradybaena brevispira]]'', ''[[Bradybaena circulus]]''<ref name="Asaro"/> ''[[Bradybaena ravida]]'', ''[[Bradybaena similaris]]'', ''[[Plectotropis appanata]]'' and ''[[Parmarion martensi]]'' from Okinawa<ref name="Asaro"/> and from Hawaii,<ref name="Hollingsworth">{{Cite doi| 10.2984/1534-6188(2007)61[457:DOPCMP]2.0.CO;2}}.</ref> ''[[Camaena cicatricosa]]'', ''[[Trichochloritis rufopila]]'', ''[[Trichochloritis hungerfordianus]]'' and ''[[Cyclophorus (gastropod)|Cyclophorus]]'' spp.<ref name="airforcemedicine 2001">(20 June 2001). "Land snail infection rates for the human parasitic nematode, ''Angiostrongylus cantonensis'' (rat lung worm) with notes on snail and parasite biology and distribution on Kadena AB, Okinawa Japan. Consultative Letter, IERA-DO-BR-CL-2001-0049." ''MEMORANDUM FOR 18 MDG/SGPM'', [[Department of the Air Force]], 11 pp. [http://airforcemedicine.afms.mil/idc/groups/public/documents/afms/ctb_020408.pdf PDF].</ref> | ||
* freshwater snails: ''[[Pila (gastropod)|Pila]]'' spp.,<ref name="Lv2009"/> ''[[Pomacea canaliculata]]'', | * freshwater snails: ''[[Pila (gastropod)|Pila]]'' spp.,<ref name="Lv2009"/> ''[[Pomacea canaliculata]]'',<ref name="Lv2009">{{Cite PMID|19771154}}</ref> ''[[Cipangopaludina chinensis]]'', ''[[Bellamya aeruginosa]]'' and ''[[Bellamya quadrata]]'' | ||
* slugs: ''[[Limax maximus]]'',<ref>{{Cite pmid|14558868}}</ref> ''[[Limax flavus]]'' | * slugs: ''[[Limax maximus]]'',<ref>{{Cite pmid|14558868}}</ref> ''[[Limax flavus]]'' ''[[Deroceras laeve]]'',<ref name="Högger 2003"/> ''[[Deroceras reticulatum]]'',<ref name="Högger 2003"/> ''[[Veronicella alte]]'',<ref name="Asaro"/> =? ''[[Laevicaulis alte]]'',<ref name="Högger 2003"/> ''[[Sarasinula plebeia]]''<!-- listed as Vaginulus plebeius-->,<ref name="Högger 2003">Högger C. H. (update 25 March 2003). "Antagonists of Slugs and Snails. A Bibliography of Sources and a List of Citations grouped according to Taxon of the Antagonists". [http://web.archive.org/web/20071214020100/http://homepage.sunrise.ch/mysunrise/choegger/Slugs/Antagonists.html in web Archive].</ref> ''[[Vaginulus yuxjsjs]]'', ''[[Lehmannia valentiana]]'',<ref name="Asaro"/> ''[[Phiolomycus bilineatus]]'', ''[[Macrochlamys loana]]'', ''[[Meghimatium bilineatum]]'' and probably other species of slugs. | ||
Definitive host of ''Angiostrongylus cantonensis'' include wild rodents, especially the brown rat (''[[Rattus norvegicus]]'')<ref name="Lindo"/> and the black rat (''[[Rattus rattus]]'').<ref name="Lindo"/> | Definitive host of ''Angiostrongylus cantonensis'' include wild rodents, especially the brown rat (''[[Rattus norvegicus]]'')<ref name="Lindo"/> and the black rat (''[[Rattus rattus]]'').<ref name="Lindo"/> | ||
| Line 62: | Line 62: | ||
Paratenic hosts of ''Angiostrongylus cantonensis'' include: predatory land flatworm ''[[Platydemus manokwari]]''<ref name="Asaro">Asato R., Taira K., Nakamura M., Kudaka J., Itokazu K. & Kawanaka M. (2004) "[http://www.nih.go.jp/niid/JJID/57/184.pdf Changing Epidemiology of Angiostrongyliasis Cantonensis in Okinawa Prefecture, Japan]". ''[[Japanese Journal of Infectious Diseases]]'' '''57''': 184-186. [http://www.nih.go.jp/JJID/57/184.html article] </ref> and amphibians ''[[Bufo asiaticus]]'',<ref name="Asaro"/> ''[[Rana catesbeiana]]'',<ref name="Asaro"/> ''[[Rhacophorus leucomystax]]''<ref name="Asaro"/> and ''[[Rana limnocharis]]''.<ref name="Asaro"/> | Paratenic hosts of ''Angiostrongylus cantonensis'' include: predatory land flatworm ''[[Platydemus manokwari]]''<ref name="Asaro">Asato R., Taira K., Nakamura M., Kudaka J., Itokazu K. & Kawanaka M. (2004) "[http://www.nih.go.jp/niid/JJID/57/184.pdf Changing Epidemiology of Angiostrongyliasis Cantonensis in Okinawa Prefecture, Japan]". ''[[Japanese Journal of Infectious Diseases]]'' '''57''': 184-186. [http://www.nih.go.jp/JJID/57/184.html article] </ref> and amphibians ''[[Bufo asiaticus]]'',<ref name="Asaro"/> ''[[Rana catesbeiana]]'',<ref name="Asaro"/> ''[[Rhacophorus leucomystax]]''<ref name="Asaro"/> and ''[[Rana limnocharis]]''.<ref name="Asaro"/> | ||
In 2004, a captive [[Yellow-tailed Black Cockatoo]] (''Calyptorhynchus funereus'') and two free-living [[Tawny Frogmouth]]s (''Podargus strigoides'') suffering neurological symptoms were shown to have the parasite. They were the first non-mammalian hosts discovered for the organism. | In 2004, a captive [[Yellow-tailed Black Cockatoo]] (''Calyptorhynchus funereus'') and two free-living [[Tawny Frogmouth]]s (''Podargus strigoides'') suffering neurological symptoms were shown to have the parasite. They were the first non-mammalian hosts discovered for the organism. | ||
==Transmission== | ==Transmission== | ||
| Line 70: | Line 70: | ||
==Incubation period== | ==Incubation period== | ||
The incubation period in humans is usually from 1 week to 1 month after infection, and can be as long as 47 days. | The incubation period in humans is usually from 1 week to 1 month after infection, and can be as long as 47 days. This interval varies, since humans are intermediate hosts and, the life cycle does not continue predictably as it would in a rat. | ||
==Eosinophilic meningitis== | ==Eosinophilic meningitis== | ||
| Line 76: | Line 76: | ||
Although the clinical disease caused by Angiostrongylus invasion into the central nervous system is commonly referred to as "eosinophilic meningitis" the actual pathophysiology is of a meningoencephalitis with invasion not just of the meninges, or superficial lining of the brain, but also deeper brain tissue. Initial invasion through the lining of the brain, the meninges, may cause a typical inflammation of the meninges and a classic meningitis picture of headache, stiff neck and often fever. The parasites subsequently invade deeper into the brain tissue, causing specific localizing neurologic symptoms depending on where in the brain [[parenchyma]] they migrate. Neurologic findings and symptoms wax and wane as initial damage is done by the physical in-migration of the worms and secondary damage is done by the inflammatory response to the presence of dead and dying worms. This inflammation can lead in the short term to paralysis, bladder dysfunction, visual disturbance and coma and in the long term to permanent nerve damage, mental retardation, nerve damage, permanent brain damage or death. | Although the clinical disease caused by Angiostrongylus invasion into the central nervous system is commonly referred to as "eosinophilic meningitis" the actual pathophysiology is of a meningoencephalitis with invasion not just of the meninges, or superficial lining of the brain, but also deeper brain tissue. Initial invasion through the lining of the brain, the meninges, may cause a typical inflammation of the meninges and a classic meningitis picture of headache, stiff neck and often fever. The parasites subsequently invade deeper into the brain tissue, causing specific localizing neurologic symptoms depending on where in the brain [[parenchyma]] they migrate. Neurologic findings and symptoms wax and wane as initial damage is done by the physical in-migration of the worms and secondary damage is done by the inflammatory response to the presence of dead and dying worms. This inflammation can lead in the short term to paralysis, bladder dysfunction, visual disturbance and coma and in the long term to permanent nerve damage, mental retardation, nerve damage, permanent brain damage or death. | ||
Eosinophilic meningitis is commonly defined by the increased number of eosinophils in the cerebrospinal fluid (CSF). In most cases, Eosinophil levels rise to 10 or more eosinophils per μL in the cerebrospinal fluid, accounting for at least 10% of the total CSF leukocyte count. | Eosinophilic meningitis is commonly defined by the increased number of eosinophils in the cerebrospinal fluid (CSF). In most cases, Eosinophil levels rise to 10 or more eosinophils per μL in the cerebrospinal fluid, accounting for at least 10% of the total CSF leukocyte count. The chemical analysis of the CSF typically resembles the findings in "[[aseptic meningitis]]" with slightly elevated protein levels, normal glucose levels and negative bacterial cultures. Presence of a significantly decreased glucose on CSF analysis is an indicator of severe meningoencephalitis and may indicate a poor prognosis. Initial CSF analysis early in the disease process may occasionally show no increase of eosinophils only to have classical increases in eosinophils in subsequest spinal fluid analysis. Caution should be advised in using eosinophilic meningitis as the only criteria for diagnosing angiostrongylus infestation in someone with classic symptoms as the disease evolves with the migration of the worms into the central nervous system. | ||
Eosinophils are specialized white blood cells of the granulocytic cell line which contain granules in their cytoplasm. These granules contain proteins that are toxic to parasites. When these granules degranulate, or break down, chemicals are released that combat parasites such as ''A. cantonensis''. Eosinophils, which are located throughout the body, are guided to sites of inflammation by chemokines when the body is infested with parasites such as ''A. cantonensis''. Once at the site of inflammation, Type 2 cytokines are released from helper T cells, which communicate with the Eosinophils, signaling them to activate. Once activated, eosinophils can begin the process of degranulation, releasing their toxic proteins in the fight against the foreign parasite. | Eosinophils are specialized white blood cells of the granulocytic cell line which contain granules in their cytoplasm. These granules contain proteins that are toxic to parasites. When these granules degranulate, or break down, chemicals are released that combat parasites such as ''A. cantonensis''. Eosinophils, which are located throughout the body, are guided to sites of inflammation by chemokines when the body is infested with parasites such as ''A. cantonensis''. Once at the site of inflammation, Type 2 cytokines are released from helper T cells, which communicate with the Eosinophils, signaling them to activate. Once activated, eosinophils can begin the process of degranulation, releasing their toxic proteins in the fight against the foreign parasite. | ||
==References== | ==References== | ||
Latest revision as of 16:01, 10 August 2015
|
Angiostrongyliasis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Angiostrongylus cantonensis On the Web |
|
American Roentgen Ray Society Images of Angiostrongylus cantonensis |
|
Risk calculators and risk factors for Angiostrongylus cantonensis |
| style="background:#Template:Taxobox colour;"|Template:Taxobox name | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
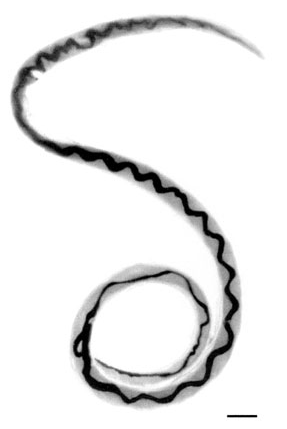 Adult female worm of Angiostrongylus cantonensis with characteristic barber-pole appearance (anterior end of worm is to the top). Scale bar is 1 mm.
| ||||||||||||||
| style="background:#Template:Taxobox colour;" | Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Angiostrongylus cantonensis (Chen, 1935) |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Angiostrongylus cantonensis is a parasitic nematode (roundworm) that causes Angiostrongyliasis, the most common cause of eosinophilic meningitis in Southeast Asia and the Pacific Basin. The nematode commonly resides in the pulmonary arteries of rats, giving it the nickname the rat lungworm. Snails are the primary intermediate hosts, where larvae develop until they are infective.
Humans are incidental hosts of this roundworm, and may become infected through ingestion of larvae in raw or undercooked snails or other vectors, or from contaminated water and vegetables. The larvae are then transported via the blood to the central nervous system (CNS), where they are the most common cause of eosinophilic meningitis, a serious condition that can lead to death or permanent brain and nerve damage. Identified in 1964, Angiostrongyliasis is an infection of increasing public health importance as globalization contributes to the geographic spread of the disease.
Infectious agent
Angiostrongylus cantonensis is a helminth of the phylum Nematoda, order Strongylida, and superfamily Metastrongyloidea. Nematodes are roundworms characterized by a tough outer cuticle, unsegmented bodies, and a fully developed gastrointestinal tract. The order Strongylida includes hookworms and lungworms. Metastrongyloidea are characterized as long, slender, threadlike worms that reside in the lungs of the definitive host.[1] Angiostrongylus costaricensis is a closely related worm that causes intestinal Angiostrongyliasis in Central and South America.
A. cantonensis is a nematode roundworm with 3 outer protective collagen layers, and a simple stomal opening or mouth with no lips or buccal cavity leading to a fully developed gastrointestinal tract. Males have a small copulatory bursa at the posterior. Females have a “barber pole” shape down the middle of the body, which is created by the twisting together of the intestine and uterine tubules. The worms are long and slender - males are 15.9-19 mm in length, and females are 21-25 mm in length.[2]
Life cycle
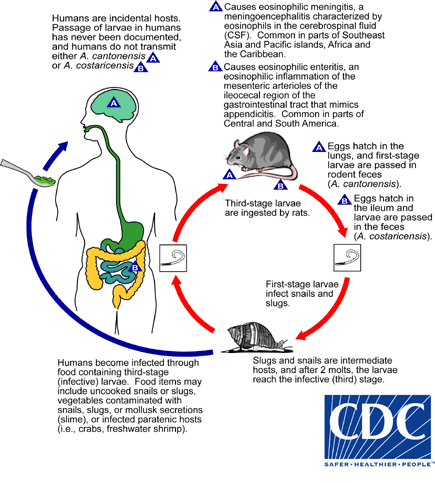
The adult form of A. cantonensis resides in the pulmonary arteries of rodents, where it reproduces. After the eggs hatch in the arteries, larvae migrate up the pharynx and are then swallowed again by the rodent and passed in the stool. These first stage larvae then penetrate or are swallowed by snail intermediate hosts, where they transform into second stage larvae and then into third stage infective larvae. Humans and rats acquire the infection when they ingest contaminated snails or paratenic (transport) hosts including prawns, crabs, and frogs, or raw vegetables containing material from these intermediate and paratenic hosts. After passing through the gastrointestinal tract, the worms enter circulation. In rats, the larvae then migrate to the meninges and develop for about a month before migrating to the pulmonary arteries, where they fully develop into adults.
Humans are incidental hosts; the larvae cannot reproduce in humans and therefore humans do not contribute to the A. cantonensis life cycle. In humans, the circulating larvae migrate to the meninges, but do not move on to the lungs. Sometimes the larvae will develop into the adult form in the brain and CSF, but they quickly die, inciting the inflammatory reaction that causes symptoms of infection.
-
Angiostrongyliasis Life Cycle
Adapted from CDC
Reservoirs
Rats are the definitive host and the main reservoir for A. cantonensis, though other small mammals may also become infected. While angiostrongylus can infect humans, humans do not act as reservoirs since the worm cannot reproduce in humans and therefore humans cannot contribute to their life cycle.
Vectors
A. cantonensis has many vectors, with the most common being several species of snails, including the giant African land snail (Achatina fulica) in the Pacific islands and snails of the genus Pila in Thailand and Malaysia. The golden apple snail, A. canaliculatus, is the most important vector in areas of China. Freshwater prawns, crabs, or other paratenic, or transport, hosts can also act as vectors.
Hosts
Intermediate hosts of larvae of for Angiostrongylus cantonensis include:
- land snails: Thelidomus aspera from Jamaica,[3] Achatina fulica,[4][5][6] Satsuma mercatoria,[5][6] Acusta despecta,[5][6] Bradybaena brevispira, Bradybaena circulus[5] Bradybaena ravida, Bradybaena similaris, Plectotropis appanata and Parmarion martensi from Okinawa[5] and from Hawaii,[7] Camaena cicatricosa, Trichochloritis rufopila, Trichochloritis hungerfordianus and Cyclophorus spp.[6]
- freshwater snails: Pila spp.,[4] Pomacea canaliculata,[4] Cipangopaludina chinensis, Bellamya aeruginosa and Bellamya quadrata
- slugs: Limax maximus,[8] Limax flavus Deroceras laeve,[9] Deroceras reticulatum,[9] Veronicella alte,[5] =? Laevicaulis alte,[9] Sarasinula plebeia,[9] Vaginulus yuxjsjs, Lehmannia valentiana,[5] Phiolomycus bilineatus, Macrochlamys loana, Meghimatium bilineatum and probably other species of slugs.
Definitive host of Angiostrongylus cantonensis include wild rodents, especially the brown rat (Rattus norvegicus)[3] and the black rat (Rattus rattus).[3]
Paratenic hosts of Angiostrongylus cantonensis include: predatory land flatworm Platydemus manokwari[5] and amphibians Bufo asiaticus,[5] Rana catesbeiana,[5] Rhacophorus leucomystax[5] and Rana limnocharis.[5]
In 2004, a captive Yellow-tailed Black Cockatoo (Calyptorhynchus funereus) and two free-living Tawny Frogmouths (Podargus strigoides) suffering neurological symptoms were shown to have the parasite. They were the first non-mammalian hosts discovered for the organism.
Transmission
Transmission of the parasite is usually from eating raw or undercooked snails or other vectors. Infection is also frequent from ingestion of contaminated water or unwashed salad that may contain small snail and slugs, or have been contaminated by them. Therefore it is very important to avoid raw snails, wash and cook vegetables thoroughly, and avoid open water sources that may be contaminated.
Incubation period
The incubation period in humans is usually from 1 week to 1 month after infection, and can be as long as 47 days. This interval varies, since humans are intermediate hosts and, the life cycle does not continue predictably as it would in a rat.
Eosinophilic meningitis
Although the clinical disease caused by Angiostrongylus invasion into the central nervous system is commonly referred to as "eosinophilic meningitis" the actual pathophysiology is of a meningoencephalitis with invasion not just of the meninges, or superficial lining of the brain, but also deeper brain tissue. Initial invasion through the lining of the brain, the meninges, may cause a typical inflammation of the meninges and a classic meningitis picture of headache, stiff neck and often fever. The parasites subsequently invade deeper into the brain tissue, causing specific localizing neurologic symptoms depending on where in the brain parenchyma they migrate. Neurologic findings and symptoms wax and wane as initial damage is done by the physical in-migration of the worms and secondary damage is done by the inflammatory response to the presence of dead and dying worms. This inflammation can lead in the short term to paralysis, bladder dysfunction, visual disturbance and coma and in the long term to permanent nerve damage, mental retardation, nerve damage, permanent brain damage or death.
Eosinophilic meningitis is commonly defined by the increased number of eosinophils in the cerebrospinal fluid (CSF). In most cases, Eosinophil levels rise to 10 or more eosinophils per μL in the cerebrospinal fluid, accounting for at least 10% of the total CSF leukocyte count. The chemical analysis of the CSF typically resembles the findings in "aseptic meningitis" with slightly elevated protein levels, normal glucose levels and negative bacterial cultures. Presence of a significantly decreased glucose on CSF analysis is an indicator of severe meningoencephalitis and may indicate a poor prognosis. Initial CSF analysis early in the disease process may occasionally show no increase of eosinophils only to have classical increases in eosinophils in subsequest spinal fluid analysis. Caution should be advised in using eosinophilic meningitis as the only criteria for diagnosing angiostrongylus infestation in someone with classic symptoms as the disease evolves with the migration of the worms into the central nervous system.
Eosinophils are specialized white blood cells of the granulocytic cell line which contain granules in their cytoplasm. These granules contain proteins that are toxic to parasites. When these granules degranulate, or break down, chemicals are released that combat parasites such as A. cantonensis. Eosinophils, which are located throughout the body, are guided to sites of inflammation by chemokines when the body is infested with parasites such as A. cantonensis. Once at the site of inflammation, Type 2 cytokines are released from helper T cells, which communicate with the Eosinophils, signaling them to activate. Once activated, eosinophils can begin the process of degranulation, releasing their toxic proteins in the fight against the foreign parasite.
References
- ↑ "Helminth Taxonomy - Phylum Nematoda", Schistosomiasis Research Group, accessed 26 February 2009.
- ↑ http://animaldiversity.ummz.umich.edu/site/accounts/information/Angiostrongylus_cantonensis.html, Accessed 2/26/09
- ↑ 3.0 3.1 3.2 Lindo J. F., Waugh C., Hall J., Cunningham-Myrie C., Ashley D., Eberhard M. L., Sullivan J. J., Bishop H. S., Robinson D. G., Holtz T. & Robinson R. D. (2002). "Enzootic Angiostrongylus cantonensis in Rats and Snails after an Outbreak of Human Eosinophilic Meningitis, Jamaica". Emerging Infectious Diseases 8(3): 324-326. HTM.
- ↑ 4.0 4.1 4.2 Template:Cite PMID
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 Asato R., Taira K., Nakamura M., Kudaka J., Itokazu K. & Kawanaka M. (2004) "Changing Epidemiology of Angiostrongyliasis Cantonensis in Okinawa Prefecture, Japan". Japanese Journal of Infectious Diseases 57: 184-186. article
- ↑ 6.0 6.1 6.2 6.3 (20 June 2001). "Land snail infection rates for the human parasitic nematode, Angiostrongylus cantonensis (rat lung worm) with notes on snail and parasite biology and distribution on Kadena AB, Okinawa Japan. Consultative Letter, IERA-DO-BR-CL-2001-0049." MEMORANDUM FOR 18 MDG/SGPM, Department of the Air Force, 11 pp. PDF.
- ↑ Template:Cite doi.
- ↑ PMID 14558868 (PMID 14558868)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ 9.0 9.1 9.2 9.3 Högger C. H. (update 25 March 2003). "Antagonists of Slugs and Snails. A Bibliography of Sources and a List of Citations grouped according to Taxon of the Antagonists". in web Archive.
External links
- Division of Parasitic Diseases, Centers for Disease Control and Prevention, Angiostrongylus cantonensis Infection
- Laboratory Identification of Parasites of Public Health Concern, Parasites and Health, Angiostrongyliasis
- Laboratory Identification of Parasites of Public Health Concern, Angiostrongyliasis Image Library
- Angiostrongylus+cantonensis at the US National Library of Medicine Medical Subject Headings (MeSH)
- Sydney Morning Herald story of human infection, Example of Angiostrongylus cantonensis human infection: Hard to swallow: slug-eating dare causes rare disease
- Angiostronglyus cantonensis on the UF / IFAS Featured Creatures website.