Aldolase A
| Aldolase A, fructose-bisphosphate | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
File:Fructose-1,6-bisphosphate aldolase 4ALD wpmp.png PDB rendering based on 4ALD. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | ALDOA ; ALDA; MGC10942; MGC17716; MGC17767 | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 68996 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
 | |||||||||||||
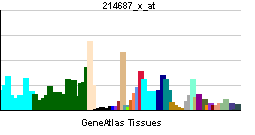 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Aldolase A is an enzyme which catalyses one of the aldol reactions: The substrate, fructose 1,6-bisphosphate (F-1,6-BP) is broken down into glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP). This reaction is a part of glycolysis. Three aldolase isozymes (A, B, and C), encoded by three different genes, are differentially expressed during development. Aldolase A is found in the developing embryo and is produced in even greater amounts in adult muscle. Aldolase A expression is repressed in adult liver, kidney and intestine and similar to aldolase C levels in brain and other nervous tissue. Aldolase A deficiency has been associated with myopathy and hemolytic anemia. Alternative splicing of this gene results in multiple transcript variants which encode the same protein.[1]
Template:Complex Enzymatic Reaction
The numbering of the carbon atoms indicates the fate of the carbons according to their position in fructose 6-phosphate.
Mechanism
In mammalian aldolase the key catylitic amino acid residues involved in the reaction are lysine and tyrosine. The tyrosine acts as an efficient hydrogen acceptor while the lysine covalently binds and stabilizes the intermediates. Many bacteria use two magnesium ions in place of the lysine.
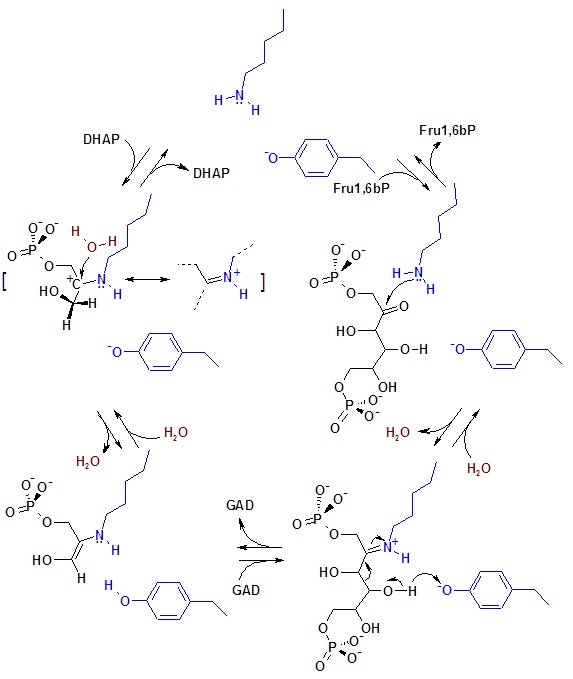
The enzyme's reactive site amino acid's side chains are shown in blue.
References
Further reading
- Pfleiderer G, Thöner M, Wachsmuth ED (1976). "Histological examination of the aldolase monomer composition of cells from human kidney and hypernephroid carcinoma". Beiträge zur Pathologie. 156 (3): 266–79. PMID 766744.
- Rehbein-Thöner M, Pfleiderer G (1977). "The changes in aldolase isoenzyme pattern during development of the human kidney and small intestine--demonstrated in organ extracts and tissue sections". Hoppe-Seyler's Z. Physiol. Chem. 358 (2): 169–80. PMID 844801.
- Wachsmuth ED (1976). "Differentiation of epithelial cells in human jejunum: localization and quantification of aminopeptidase, alkaline phosphatase and aldolase isozymes in tissue sections". Histochemistry. 48 (2): 101–9. PMID 955981.
- Lee KN, Maxwell MD, Patterson MK; et al. (1992). "Identification of transglutaminase substrates in HT29 colon cancer cells: use of 5-(biotinamido)pentylamine as a transglutaminase-specific probe". Biochim. Biophys. Acta. 1136 (1): 12–6. PMID 1353685.
- Dawson SJ, White LA (1992). "Treatment of Haemophilus aphrophilus endocarditis with ciprofloxacin". J. Infect. 24 (3): 317–20. PMID 1602151.
- Mukai T, Arai Y, Yatsuki H; et al. (1991). "An additional promoter functions in the human aldolase A gene, but not in rat". Eur. J. Biochem. 195 (3): 781–7. PMID 1999195.
- Gamblin SJ, Davies GJ, Grimes JM; et al. (1991). "Activity and specificity of human aldolases". J. Mol. Biol. 219 (4): 573–6. PMID 2056525.
- Vértessy BG, Orosz F, Ovádi J (1991). "Modulation of the interaction between aldolase and glycerol-phosphate dehydrogenase by fructose phosphates". Biochim. Biophys. Acta. 1078 (2): 236–42. PMID 2065091.
- Takasaki Y, Takahashi I, Mukai T, Hori K (1990). "Human aldolase A of a hemolytic anemia patient with Asp-128----Gly substitution: characteristics of an enzyme generated in E. coli transfected with the expression plasmid pHAAD128G". J. Biochem. 108 (2): 153–7. PMID 2229018.
- Gamblin SJ, Cooper B, Millar JR; et al. (1990). "The crystal structure of human muscle aldolase at 3.0 A resolution". FEBS Lett. 262 (2): 282–6. PMID 2335208.
- Kishi H, Mukai T, Hirono A; et al. (1988). "Human aldolase A deficiency associated with a hemolytic anemia: thermolabile aldolase due to a single base mutation". Proc. Natl. Acad. Sci. U.S.A. 84 (23): 8623–7. PMID 2825199.
- Izzo P, Costanzo P, Lupo A; et al. (1987). "A new human species of aldolase A mRNA from fibroblasts". Eur. J. Biochem. 164 (1): 9–13. PMID 3030757.
- Inagaki H, Haimoto H, Hosoda S, Kato K (1988). "Aldolase C is localized in neuroendocrine cells". Experientia. 44 (9): 749–51. PMID 3046960.
- Freemont PS, Dunbar B, Fothergill-Gilmore LA (1988). "The complete amino acid sequence of human skeletal-muscle fructose-bisphosphate aldolase". Biochem. J. 249 (3): 779–88. PMID 3355497.
- Izzo P, Costanzo P, Lupo A; et al. (1988). "Human aldolase A gene. Structural organization and tissue-specific expression by multiple promoters and alternate mRNA processing". Eur. J. Biochem. 174 (4): 569–78. PMID 3391172.
- Maire P, Gautron S, Hakim V; et al. (1988). "Characterization of three optional promoters in the 5' region of the human aldolase A gene". J. Mol. Biol. 197 (3): 425–38. PMID 3441006.
- Kukita A, Yoshida MC, Fukushige S; et al. (1987). "Molecular gene mapping of human aldolase A (ALDOA) gene to chromosome 16". Hum. Genet. 76 (1): 20–6. PMID 3570299.
- Tolan DR, Niclas J, Bruce BD, Lebo RV (1987). "Evolutionary implications of the human aldolase-A, -B, -C, and -pseudogene chromosome locations". Am. J. Hum. Genet. 41 (5): 907–24. PMID 3674018.
- Sakakibara M, Mukai T, Hori K (1985). "Nucleotide sequence of a cDNA clone for human aldolase: a messenger RNA in the liver". Biochem. Biophys. Res. Commun. 131 (1): 413–20. PMID 3840020.
- Ovádi J, Mohamed Osman IR, Batke J (1983). "Interaction of the dissociable glycerol-3-phosphate dehydrogenase and fructose-1,6-bisphosphate aldolase. Quantitative analysis by an extrinsic fluorescence probe". Eur. J. Biochem. 133 (2): 433–7. PMID 6406231.
External links
- http://pdbdev.sdsc.edu:48346/pdb/molecules/pdb50_5.html
- Fructose-Bisphosphate+Aldolase at the US National Library of Medicine Medical Subject Headings (MeSH)
- ALDOA
- EC 4.1.2.13
Template:Metabolic pathway stub
ATP
ADP
ATP
ADP
+ +
NAD++ Pi
NADH + H+
NAD++ Pi
NADH + H+ H2O
H2O ADP
ATP
2 × Pyruvate 2 × File:Pyruvat.svg
|