Procainamide
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | IV, IM, oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% (oral) |
| Protein binding | 15 to 20% |
| Metabolism | Hepatic (CYP2D6-mediated) |
| Elimination half-life | ~2.5 to 4.5 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H21N3O |
| Molar mass | 235.325 g/mol |
|
WikiDoc Resources for Procainamide |
|
Articles |
|---|
|
Most recent articles on Procainamide Most cited articles on Procainamide |
|
Media |
|
Powerpoint slides on Procainamide |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Procainamide at Clinical Trials.gov Clinical Trials on Procainamide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Procainamide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Procainamide Discussion groups on Procainamide Patient Handouts on Procainamide Directions to Hospitals Treating Procainamide Risk calculators and risk factors for Procainamide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Procainamide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
For patient information, click here
Overview
Procainamide (proe-KANE-a-mide) (INN, trade names Pronestyl®, Procan®, Procanbid®) is a pharmaceutical antiarrhythmic agent used for the medical treatment of cardiac arrhythmias, classified by the Vaughan Williams classification system as class Ia. Procanbid® will no longer be manufactured.1
It blocks open sodium (Na+) channels and prolongs the cardiac action potential (outward potassium (K+) currents may be blocked). This results in slowed conduction, and ultimately the decreased rate of rise of the action potential, which may result in widening of QRS on electrocardiogram (ECG). This drug is used for both supraventricular and ventricular arrhythmias. For example, it can be used to convert new-onset atrial fibrillation, though it is suboptimal for this purpose.
Procainamide is administered intravenously or orally. When administered intravenously, a loading dose should first be given, though care should be taken not to cause hypotension. Procainamide's active metabolite is N-acetyl procainamide, which is excreted by the kidneys and the renal system.
Adverse effects include rash, myalgia, hypersensitivity reactions (fever, agranulocytosis), Drug-Induced Lupus Erythematosus (particularly in slow-acetylators), and proarrhythmic effects (e.g., torsades de pointes). Treatment with procainamide can cause antibody production against cellular components, accounting for the systemic lupus erythematosus-like adverse reactions.
Warning
The prolonged administration of Procainamide often leads to the development of a positive anti-nuclear antibody (ANA) test, with or without symptoms of lupus erythematosus-like syndrome. If a positive ANA titer develops, the benefits versus risks of continued Procainamide therapy should be assessed.
Procainamide Description
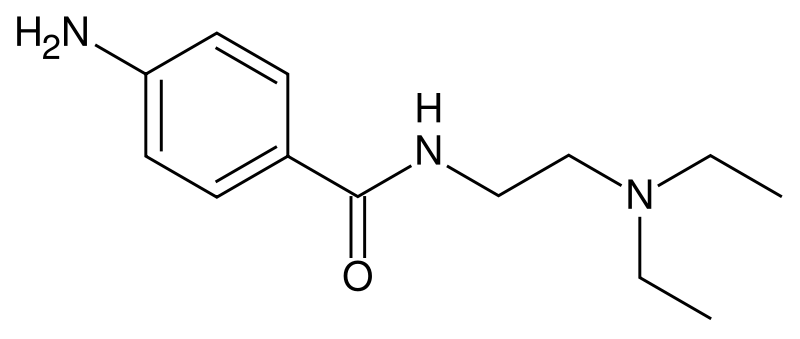
Procainamide hydrochloride, a Group 1A cardiac antiarrhythmic drug, is p-amino-N-[2-(diethylamino)ethyl]-benzamide monohydrochloride. It differs from procaine which is the p-aminobenzoyl ester of 2-(diethylamino)-ethanol. Procainamide as the free base has a PKa of 9.23; the monohydrochloride is very soluble in water. Its structural formula is:
Procainamide hydrochloride, is a white to tan odorless, crystalline salt that is readily soluble in water.
Procainamide hydrochloride is available as 250 mg, 375 mg, or 500 mg capsules for oral administration and contains the following inactive ingredients: anhydrous lactose, glacial acetic acid, magnesium stearate, methylparaben, pregelatinized starch, propylparaben, silicon dioxide, sodium lauryl sulfate, stearic acid and talc. Additional inactive ingredients in the gelatin capsule include (250 mg and 500 mg) FD&C Yellow No. 6, D&C Yellow No. 10, titanium dioxide; (375 mg) FD&C Yellow No. 6, titanium dioxide. The 250 mg capsule also contains glycerine.
Procainamide - Clinical Pharmacology
Procainamide (PA) increases the effective refractory period of the atria, and to a lesser extent the bundle of His-Purkinje system and ventricles of the heart. It reduces impulse conduction velocity in the atria, His-Purkinje fibers, and ventricular muscle, but has variable effects on the atrioventricular (A-V) node, a direct slowing action and a weaker vagolytic effect which may speed A-V conduction slightly. Myocardial excitability is reduced in the atria, Purkinje fibers, papillary muscles, and ventricles by an increase in the threshold for excitation, combined with inhibition of ectopic pacemaker activity by retardation of the slow phase of diastolic depolarization, thus decreasing automaticity especially in ectopic sites. Contractility of the undamaged heart is usually not affected by therapeutic concentrations, although slight reduction of cardiac output may occur, and may be significant in the presence of myocardial damage. Therapeutic levels of PA may exert vagolytic effects and produce slight acceleration of heart rate, while high or toxic concentrations may prolong A-V conduction time or induce A-V block, or even cause abnormal automaticity and spontaneous firing, by unknown mechanisms.
The electrocardiogram may reflect these effects by showing slight sinus tachycardia (due to anticholinergic action) and widened QRS complexes and, less regularly, prolonged Q-T and P-R intervals (due to longer systole and slower conduction), as well as some decreases in QRS and T wave amplitude. These direct effects of PA on electrical activity, conduction, responsiveness, excitability and automaticity are characteristic of Group 1A antiarrhythmic agent, the prototype for which is quinidine; PA effects are very similar. However, PA has weaker vagal blocking action than does quinidine, does not induce alpha-adrenergic blockade, and is less depressing to cardiac contractility.
Ingested PA is resistant to digestive hydrolysis, and the drug is well absorbed from the entire small intestine surface, but individual patients vary in their completeness of absorption of PA. Following oral administration of Procainamide hydrochloride, plasma PA levels peak in approximately 45 to 120 minutes. About 15 to 20 percent of PA is reversibly bound to plasma proteins, and considerable amounts are more slowly and reversibly bound to tissues of the heart, liver, lung, and kidney. The apparent volume of distribution eventually reaches about 2 liters per kilogram body weight with a half-time of approximately five minutes. While PA has been shown in the dog to cross the blood-brain barrier, it did not concentrate in the brain at levels higher than in plasma. It is not known if PA crosses the placenta. Plasma esterases are far less active in hydrolysis of PA than of procaine. The half-time for elimination is three to four hours in patients with normal renal function, but reduced creatinine clearance and advancing age each prolong the half-time of elimination of PA.
A significant fraction of the circulating PA may be metabolized in hepatocytes to N-acetylProcainamide (NAPA), ranging from 16 to 21 percent of an administered dose in “slow acetylators” to 24 to 33 percent in “fast-acetylators”. Since NAPA also has significant antiarrhythmic activity and somewhat slower renal clearance than PA, both hepatic acetylation rate capability and renal function, as well as age, have significant effects on the effective biological half-time of therapeutic action of administered PA and NAPA derivative. Trace amounts may be excreted in the urine as free and conjugated p-aminobenzoic acid, 30 to 60 percent as unchanged PA, and 6 to 52 percent as the NAPA derivative. Both PA and NAPA are eliminated by active tubular secretion as well as by glomerular filtration. Action of PA on the central nervous system is not prominent, but high plasma concentrations may cause tremors. While therapeutic plasma levels for PA have been reported to be 3 to 10 µg/mL, certain patients such as those which sustained ventricular tachycardia may need higher levels for adequate control. This may justify the increased risk of toxicity (see OVERDOSAGE). Where programmed ventricular stimulation has been used to evaluate efficacy of PA in preventing recurrent ventricular tachyarrhythmias, higher plasma levels (mean, 13.6 µg/mL) of PA were found necessary for adequate control.
Indications and Usage for Procainamide
Procainamide hydrochloride capsules are indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgement of the physician, are life-threatening. Because of the proarrhythmic effects of Procainamide, its use with lesser arrhythmias is generally not recommended. Treatment of patients with asymptomatic ventricular premature contractions should be avoided.
Initiation of Procainamide treatment, as with other antiarrhythmic agents used to treat life-threatening arrhythmias, should be carried out in the hospital.
Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias.
Because Procainamide has the potential to produce serious hematological disorders (0.5 percent) particularly leukopenia or agranulocytosis (sometimes fatal), its use should be reserved for patients in whom, in the opinion of the physician, the benefits of treatment clearly outweigh the risks. (See WARNINGS and Boxed Warning.)
Contraindications
Complete heart block
Procainamide should not be administered to patients with complete heart block because of its effects in suppressing nodal or ventricular pacemakers and the hazard of asystole. It may be difficult to recognize complete heart block in patients with ventricular tachycardia, but if significant slowing of ventricular rate occurs during PA treatment without evidence of A-V conduction appearing, PA should be stopped. In cases of second degree A-V block or various types of hemiblock, PA should be avoided or discontinued because of the possibility of increased severity of block, unless the ventricular rate is controlled by an electrical pacemaker.
Idiosyncratic hypersensitivity
ln patients sensitive to procaine or other ester-type local anesthetics, cross sensitivity to PA is unlikely; however, it should be borne in mind, and PA should not be used if it produces acute allergic dermatitis, asthma, or anaphylactic symptoms.
Lupus Erythematosus
An established diagnosis of systemic lupus erythematosus is a contraindication to PA therapy, since aggravation of symptoms is highly likely.
Torsade de Pointes
In the unusual ventricular arrhythmia called “les torsades de pointes” (Twistings of the points), characterized by alternation of one or more ventricular premature beats in the directions of the QRS complexes on ECG in persons with prolonged Q-T and often enhanced U waves, Group 1A anti-arrhythmic drugs are contraindicated. Administration of PA in such cases may aggravate this special type of ventricular extrasystole or tachycardia instead of suppressing it.
Warnings
Mortality
In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-centered, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than six days but less than two years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7 %) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to matched placebo-treated group (3.0 %). The average duration of treatment with encainide or flecainide in this study was ten months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarctions) is uncertain. Considering the known proarrhythmic properties of Procainamide and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of Procainamide as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias.
Blood Dyscrasias
Agranulocytosis, bone marrow depression, neutropenia, hypoplastic anemia and thrombocytopenia in patients receiving Procainamide hydrochloride have been reported at a rate of approximately 0.5%. Most of these patients received Procainamide within the recommended dosage range. Fatalities have occurred (with approximately 20-25 percent mortality in reported cases of agranulocytosis). Since most of these events have been noted during the first 12 weeks of therapy, it is recommended that complete blood counts including white cell, differential and platelet counts be performed at weekly intervals for the first three months of therapy; and periodically thereafter. Complete blood counts should be performed promptly if the patient develops any signs of infection (such as fever, chills, sore throat or stomatitis), bruising or bleeding. If any of these hematological disorders are identified, Procainamide therapy should be discontinued. Blood counts usually return to normal within one month of discontinuation. Caution should be used in patients with pre-existing marrow failure or cytopenia of any type. (See ADVERSE REACTIONS).
Digitalis intoxication
Caution should be exercised in the use of Procainamide in arrhythmias associated with digitalis intoxication. Procainamide can suppress digitalis-induced arrythmias; however, if there is concomitant marked disturbance of atrioventricular conduction, additional depression of conduction and ventricular asystole or fibrillation may result. Therefore, use of Procainamide should be considered only if discontinuation of digitalis, and therapy with potassium, lidocaine, or phenytoin are ineffective.
First degree heart block
Caution should be exercised also if the patient exhibits or develops first degree heart block while taking PA, and dosage reduction is advised in such cases. If the block persists despite dosage reduction, continuation of PA administration must be evaluated on the basis of current benefit versus risk of increased heart block.
Predigitalization for atrial flutter or fibrillation
Patients with atrial flutter or fibrillation should be cardioverted or digitalized prior to PA administration to avoid enhancement of A-V conduction which may result in ventricular rate acceleration beyond tolerable limits. Adequate digitalization reduces but does not eliminate the possibility of sudden increase in ventricular rate as the atrial rate is slowed by PA in these arrhythmias.
Congestive heart failure
For patients in congestive heart failure, and those with acute ischemic heart disease or cardiomyopathy, caution should be used in PA therapy, since even slight depression of myocardial contractility may further reduce cardiac output of the damaged heart.
Concurrent other antiarrhythmic agents
Concurrent use of PA with other Group 1A antiarrhythmic agents such as quinidine or disopyramide may produce enhanced prolongation of conduction or depression of contractility and hypotension, especially in patients with cardiac decompensation. Such use should be reserved for patients with serious arrhythmias unresponsive to a single drug and employed only if close observation is possible.
Renal insufficiency
Renal insufficiency may lead to accumulation of high plasma levels from conventional oral doses of PA, with effects similar to those of overdosage (see OVERDOSAGE), unless dosage is adjusted for the individual patient.
Myasthenia gravis
Patients with myasthenia gravis may show worsening of symptoms from PA due to its procaine-like effect on diminishing acetylcholine release at skeletal muscle motor nerve endings, so that PA administration may be hazardous without optimal adjustment of anticholinesterase medications and other precautions.
Precautions
General
Immediately after initiation of PA therapy, patients should be closely observed for possible hypersensitivity reactions, especially if procaine or local anesthetic sensitivity is suspected, and for muscular weakness if myasthenia gravis is a possibility.
In conversion of atrial fibrillation to normal sinus rhythm by any means, dislodgement of mural thrombi may lead to embolization, which should be kept in mind. After a day or so, steady state plasma PA levels are produced following regular oral administration of a given dose of Procainamide Hydrochloride Capsules at set intervals, with peak plasma concentrations at about 45 to 120 minutes after each dose. After achieving and maintaining therapeutic plasma concentrations and satisfactory electrocardiographic and clinical responses, continued frequent periodic monitoring of vital signs and electrocardiograms is advised. If evidence of QRS widening of more than 25 percent or marked prolongation of the Q-T interval occurs, concern for overdosage is appropriate, and reduction in dosage, is advisable if a 50 percent increase occurs. Elevated serum creatinine or urea nitrogen, reduced creatinine clearance, or history of renal insufficiency, as well as use in older patients (over age 50), provide grounds to anticipate that less than the usual dosage and longer time intervals between doses may suffice, since the urinary elimination of PA and NAPA may be reduced, leading to gradual accumulation beyond normally-predicted amounts. If facilities are available for measurement of plasma PA and NAPA, or acetylation capability, individual dose adjustment for optimal therapeutic levels may be easier, but close observation of clinical effectiveness is the most important criterion.
In the longer term, periodic complete blood counts are useful to detect possible idosyncratic hematologic effects of PA on neutrophil, platelet or red cell homeostasis; agranulocytosis has been reported to occur occasionally in patients on long-term PA therapy. A rising titer of serum ANA may precede clinical symptoms of the lupoid syndrome (see Boxed Warning above DESCRIPTION section, and ADVERSE REACTIONS). If the lupus erythematosus-like syndrome develops in a patient with recurrent life threatening arrhythmias not controlled by other agents, corticosteroid suppressive therapy may be used concomitantly with PA. Since the PA-induced lupoid syndrome rarely includes the dangerous pathologic renal changes, PA therapy may not necessarily have to be stopped unless the symptoms of serositis and the possibility of further lupoid effects are of greater risk than the benefit of PA in controlling arrhythmias. Patients with rapid acetylation capability are less likely to develop the lupoid syndrome after prolonged PA therapy.
References
1. http://www.fda.gov/cder/drug/shortages/procanletter.pdf
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Antiarrhythmic agents
- Drugs
- Cardiology