Lopressor/adverse reactions
 | |
| Clinical data | |
|---|---|
| Trade names | Lopressor, Toprol-xl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682864 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Elimination half-life | 3-7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
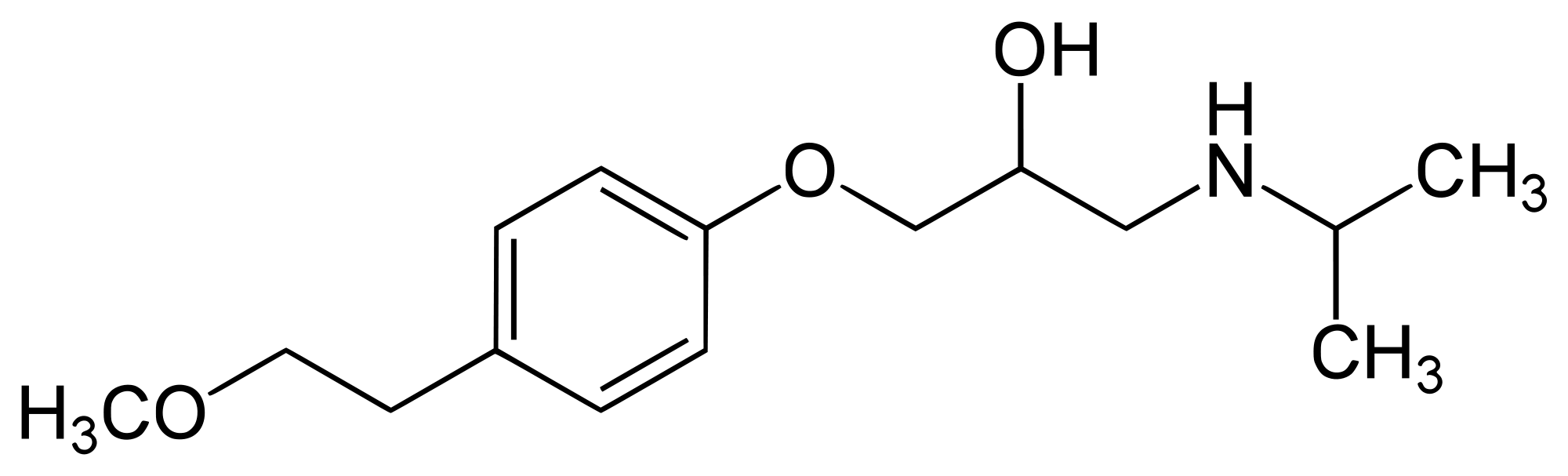
| Formula | C15H25NO3 |
| Molar mass | 267.364 g/mol |
| 3D model (JSmol) | |
| Melting point | 120 °C (248 °F) |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Adverse Reactions
Hypertension and Angina
Most adverse effects have been mild and transient.
Central Nervous System: Tiredness and dizziness have occurred in about 10 of 100 patients. Depression has been reported in about 5 of 100 patients. Mental confusion and short-term memory loss have been reported. Headache, nightmares, and insomnia have also been reported.
Cardiovascular: Shortness of breath and bradycardia have occurred in approximately 3 of 100 patients. Cold extremities; arterial insufficiency, usually of the Raynaud type; palpitations; congestive heart failure; peripheral edema; and hypotension have been reported in about 1 of 100 patients. Gangrenein patients with pre-existing severe peripheral circulatory disorders has also been reported very rarely. (See CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS.)
Respiratory: Wheezing (bronchospasm) and dyspnea have been reported in about 1 of 100 patients (see WARNINGS). Rhinitis has also been reported.
Gastrointestinal: Diarrhea has occurred in about 5 of 100 patients. Nausea, dry mouth, gastric pain, constipation, flatulence, and heartburn have been reported in about 1 of 100 patients. Vomiting was a common occurrence. Postmarketing experience reveals very rare reports of hepatitis, jaundice and non-specific hepatic dysfunction. Isolated cases of transaminase, alkaline phosphatase, and lactic dehydrogenase elevations have also been reported.
Hypersensitive Reactions: Pruritus or rash have occurred in about 5 of 100 patients. Very rarely, photosensitivity and worsening of psoriasis has been reported.
Miscellaneous: Peyronie’s disease has been reported in fewer than 1 of 100,000 patients. Musculoskeletal pain, blurred vision, and tinnitus have also been reported.
There have been rare reports of reversible alopecia, agranulocytosis, and dry eyes. Discontinuation of the drug should be considered if any such reaction is not otherwise explicable.
There have been very rare reports of weight gain, arthritis, and retroperitoneal fibrosis (relationship to Lopressor has not been definitely established).
The oculomucocutaneous syndrome associated with the beta blocker practolol has not been reported with Lopressor.
Myocardial Infarction
Central Nervous System: Tiredness has been reported in about 1 of 100 patients. Vertigo, sleep disturbances, hallucinations, headache, dizziness, visual disturbances, confusion, and reduced libido have also been reported, but a drug relationship is not clear.
Cardiovascular: In the randomized comparison of Lopressor and placebo described in the CLINICAL PHARMACOLOGY section, the following adverse reactions were reported:
 |
Respiratory: Dyspnea of pulmonary origin has been reported in fewer than 1 of 100 patients.
Gastrointestinal: Nausea and abdominal pain have been reported in fewer than 1 of 100 patients.
Dermatologic: Rash and worsened psoriasis have been reported, but a drug relationship is not clear.
Miscellaneous: Unstable diabetes and claudication have been reported, but a drug relationship is not clear.
Potential Adverse Reactions
A variety of adverse reactions not listed above have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to Lopressor. Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular: Intensification of AV block (see CONTRAINDICATIONS).
Hematologic: Agranulocytosis, nonthrombocytopenic purpura, and thrombocytopenic purpura.
Hypersensitive Reactions: Fever combined with aching and sore throat, laryngospasm and respiratory distress.
Postmarketing Experience
The following adverse reactions have been reported during postapproval use of Lopressor: confusional state, an increase in blood triglycerides and a decrease in High Density Lipoprotein (HDL). Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.[1]
References
Adapted from the FDA Package Insert.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Drugs
- Cardiovascular Drugs
- Beta blockers