Stress cardiomyopathy
| Stress cardiomyopathy | |
 | |
|---|---|
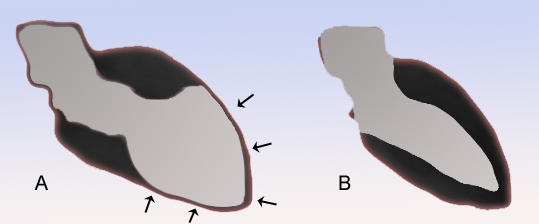
| Schematic representation of Takotsubo cardiomyopathy (A) compared to the situation in a normal person (B). | |
| ICD-9 | 429.83 |
| DiseasesDB | 33976 |
| MeSH | 054549 |
Editors-In-Chief: Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Left Ventricular Apical Ballooning Syndrome, also known as Takotsubo cardiomyopathy, or Ampulla-Shaped Cardiomyopathy. is a cardiac syndrome characterized by a reversible transient apical ventricular dysfunction. Since the cardiomyopathy is often triggered by emotional stress, such as the death of a loved one, the condition is sometimes also referred to as the Broken Heart Syndrome. In 2006, the syndrome was renamed Stress Cardiomyopathy, and was classified as an acquired cardiomyopathy. [1]
The typical presentation of someone with takotsubo cardiomyopathy is a sudden onset of congestive heart failure or chest pain associated with EKG changes suggestive of an anterior wall acute MI. During the course of evaluation of the patient, a bulging out of the left ventricular apex with a hypercontractile base of the left ventricle is often noted. It is the hallmark bulging out of the apex of the heart with preserved function of the base that earned the syndrome its name "tako tsubo", or octopus trap in Japan, where it was first described. Evaluation of individuals with takotsubo cardiomyopathy typically include a coronary angiogram, which does not reveal any significant blockages that would cause the left ventricular dysfunction. Provided that the individual survives their initial presentation, the left ventricular function improves within days to weeks.[2] Takotsubo cardiomyopathy is more commonly seen in post-menopausal women.[3] Often there is a history of a recent severe emotional or physical stress.[3]
Epidemiology and Demographics
The exact incidence is unknown, but it is estimated that apical ballooning syndrome may account for 1-2% of patients who present with an acute myocardial infarction. The cardiomyopathy appears to occur almost exclusively in post-menopausal women, though a few cases have been reported in younger women and males.
The syndrome has been reported to occur after earthquakes, [4] after non-cardiac surgery, [5] and in patients with noncardiac medical emergencies. [6]
Pathophysiology
The etiology of stress cardiomyopathy appears to involve the response of the myocardium to a hyperadrenergic state. The syndrome is often preceded by significant emotional and physical stress. Serum catecholamines may be markedly elevated in patients with stress cardiomyopathy with levels greater than seen in patients with thrombotic ST elevation myocardial infarction or congestive heart failure, though this is not always present. Thus, it has been postulated that catecholamine excess contributes at least in part to the pathophysiology, and that catecholamines may cause direct myonecrosis. [7]
Several other pathophysiologic mechanisms have been proposed. Lyon et al have hypothesized that the syndrome is a form of myocardial stunning that is mediated by epinephrine. [8] These authors hypothesize that high levels of circulating epinephrine observed in the syndrome trigger a switch in intracellular signal trafficking.
In particular, they hypothesize that ventricular cardiomyocytes (particularly those at the apex), switch from G(s) protein to G(i) protein signaling via the beta(2)-adrenoceptor. One potential benefit of this switch to beta(2)-adrenoceptor-G(i) protein signaling is that this may afford protection against the proapoptotic effects of intense activation of beta(1)-adrenoceptors. On the other hand, this switch is also negatively inotropic. Because beta-adrenoceptor density is greatest at the apex of the left ventricle (455 vs 341 fmol/mg),[9] the mechanical impact of the switch is also greatest at the apical myocardium. [8]
Patients who develop the cardiomyopathy appear to have a higher prevalence of anxiety disorders preceding the event which suggests that psychosocial stress may be a predisposing factor (Summers et al. JACC 2010).
While some of the original researchers of apical ballooning suggested that spasm in multiple coronary arteries could reduce epicardial blood flow to cause transient stunning of the myocardium (Kurisu et al. American Heart Journal 2002), other researchers have shown that vasospasm is much less common than initially thought (Tsuchuhashi K et al. JACC 2001, Kawai et al. JPJ 2000, Desmet et al. Heart 2003). It has also been noted that when there is epicardial artery vasospasm, even in multiple arteries, that they do not correlate with the areas of myocardium that are hypokinetic (Abe et al. JACC 2003).
One final hypothesis is that the syndrome is due to microvascular dysfunction.
It may be that the pathophysiology is multifactorial.
Natural History
The in-hospital mortality is very low (1-2%), typically related to the underlying disease in those with physical stressors. Long term survival is good and recurrance rate is low (10%).
Diagnosis
Differential Diagnosis
Other conditions that stress cardiomyopathy should be distinguished from include:
- Acute Coronary Syndrome (common)
- Myocarditis (uncommon)
- Pheochromocytoma induced cardiomyopathy (rare)
Mayo Criteria
Mayo Clinic Criteria for Apical Ballooning Syndrome. All 4 must be present [10]:
- Transient hypokinesis, akinesis or dyskinesis of the left ventricular mid-segments with or without apical involvement. The regional wall motion abnormalities extend beyond a single epicardial vascular distribution. A stressful trigger is often, but not always present
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
- New electrocardiographic abnormalities (either ST-segment elevation and/or T- wave inversion) or modest elevation in cardiac troponin.
- Absence of pheochromocytoma and myocarditis
Symptoms and Signs
Case series looking at large groups of patients report that some patients develop apical balloon syndrome after an emotional stressor, while others have a preceding clinical stressor (such as an asthma attack or sudden illness). Roughly one third of patients have no preceding stressful event [11].
The typical presentation of patients with Apical Ballooning Syndrome is with acute chest pain or shortness of breath, and is similar to an acute coronary syndrome.
Laboratory Findings
Electrocardiogram
The EKG findings are often confused with those of an acute anterior wall myocardial infarction.[3][12] While the ECG may reveal ST-segment elevation, it may also reveal non-specific ST/T wave abnormality, usually in the precordial leads. The 12-lead ECG alone is not helpful in differentiating apical ballooning syndrome from a traditional thrombotic ST-elevation myocardial infarction. Evolutionary changes occur over 2 to 3 days that are characteristic and include resolution of the ST-segment elevation and development of diffuse and frequently deep T-wave inversion.
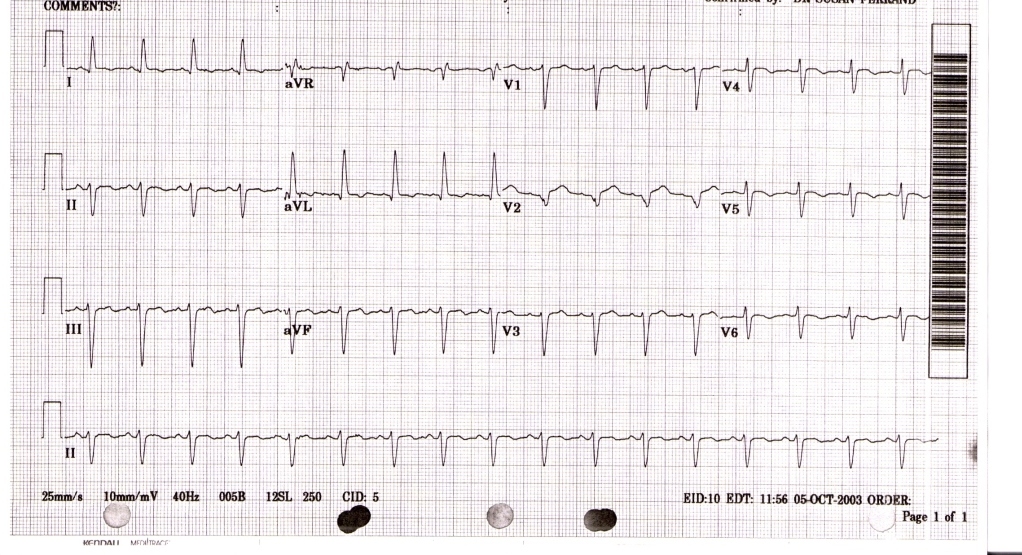
The diagnosis of takotsubo cardiomyopathy may be difficult upon presentation. The EKG findings are often confused with those found during an acute anterior wall myocardial infarction.[3][12]
Biomarker Studies
Cardiac biomarkers of myonecrosis, especially troponin, are invariably elevated.
Echocardiography

Cardiac Catheterization
Coronary angiography usually demonstrates normal coronary arteries or mild coronary atherosclerosis. The left ventriculogram usually reveals characteristic regional wall motion abnormalities which involve the mid and usually the apical segments. There is sparing of the basal systolic function, and the wall motion abnormality extends beyond the distribution of any one single coronary artery.

The diagnosis is made by the pathognomic wall motion abnormalities, in which the base of the left ventricle is contracting normally or are hyperkinetic while the remainder of the left ventricle is akinetic or dyskinetic. This is accompanied by the lack of significant coronary artery disease that would explain the wall motion abnormalities.
Magnetic Resonance Imaging
Cardiac magnetic resonance imaging is helpful in excluding a myocardial infarction due to the absence of delayed gadolinium hyperenhancement.
The MRIs below show a patients heart with apical ballooning and then later after resolution of the apical ballooning.
MRI during apical balllooning: <youtube v=23w6f71zTXI/> ____
MRI following resolution of apical ballooning: <youtube v=qE0YrlQ5d1o/>
The Various Patterns of Wall Motion Abnormalities
It should be that the wall motion abnormalities are not always anteroapical.
Diagnostic Studies
Recent research has aimed to expand the clinical understanding of cardiomyopathy. Researchers no longer consider the clinical profile to be as population-specific as previously conceived. It has been observed that, while stress cardiomyopathy may typically impact older, post-menopausal women, stress cardiomyopathy can also affect male and younger female populations. The study also determined that a stressful trigger is not a necessary component of stress cardiomyopathy and may be altogether absent. The study suggests the use of MRIs as a valuable diagnostic tool in differentiating cardiomyopathy from acute myocardial infarction and myocarditis cases.
The Eitel Study
Recent research has acknowledged the shortcoming of existing data [20]. An attempt has been made to prospectively evaluate the influence of stress cardiomyopathy. The Eitel study, conducted by Dr. Ingo Eitel of the University of Leipzig Heart Center, Germany, evaluated a population of 256 European and North American patients who presented with stress cardiomyopathy at tertiary care centers. Patients were prospectively followed up with between the first and sixth month after the acute event. The primary outcome of this study was the complete recovery of the left ventricular (LV) dysfunction. Study demographics revealed that those patients presenting with stress cardiomyopathy included members of the populations not previously thought to be afflicted with the syndrome. Post-menopausalwomen made up 81% of enrolled patients, while 8% were younger women, and 11% were men. The data suggests that stress cardiomyopathy has a much broader range of influence than originally conceived.
Similarly, though it had been previously reported that an identifiable stressful event occurred in most patients (90%) prior to onset of stress cardiomyopathy, only 71% of patients in Eitel et al.’s study experienced a clearly identifiable emotional or physical trigger. Thus, it cannot be assumed that all stress cardiomyopathy patients experience a common trigger, and a stress cardiomyopathy diagnosis cannot be discounted.
To date, the Eitel study is the largest, (multi-center) cardiovascular MRI imaging series of stress cardiomyopathy. Though more research is needed to examine this rare disease, Eitel et al have found that stress cardiomyopathy can be accurately diagnosed by identifying a typical pattern of LV dysfunction, myocardial edema, absence of significant necrosis/fibrosis, and markers of myocardial inflammation.
Of the 256 study patients, 239 patients (93%) had cardiovascular MRI data available. This data shows that there are four distinct patterns of regional ventricular ballooning: apical (82%), biventricular (34%), midventricular (17%), and basal (1%). Because patients with RV involvement tended to be older, hospitalized for longer, and have markers of heart failure, biventricular ballooning on MRI “may portend a longer and more severe course of disease compared with patients with isolated (LV) involvement.” Dysfunctions in the right ventricle are important to identify due to its effects on morbidity, treatment, and outcome. During follow up MRIs, patients exhibited normalization of LVEF (66%) and inflammatory markers in the absence of significant fibrosis in all patients.
Treatment
The treatment of stress cardiomyopathy is supportive as the condition is reversible. Initial treatment should be similar to that of an acute coronary syndrome with therapy directed at relieving myocardial ischemia with administration of aspirin, intravenous heparin and beta blockers. Once a diagnosis of stress cardiomyopathy has been confirmed and an acute coronary syndrome excluded, consideration should be given to continuing beta-blocker therapy empirically since catecholamines are suspected of contributing to the syndrome. Diuretics are effective for the treatment of congestive heart failure. Angiotensin converting enzyme inhibitors may be used if the diagnosis is uncertain, until there is complete recovery of systolic function. Insofar as the left ventricular function and apical wall motion return to normal within days or weeks, long-term anti-coagulation does not appear to be necessary.
Complications[21]
- Heart failure
- Cardiogenic shock
- Left ventricular outflow obstruction
- Mitral regurgitation[22][23]
- Ventricular arrhythmias
Prognosis
Majority of the patients completely recover within 4-8weeks. Recurrence rate is about 3%[24][25].
Additional Reading
[26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36]
References
- ↑ Maron BJ, Towbin JA, Thiene G; et al. (2006). "Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention". Circulation. 113 (14): 1807–16. doi:10.1161/CIRCULATIONAHA.106.174287. PMID 16567565.
- ↑ Akashi YJ, Barbaro G, Sakurai T, Nakazawa K, Miyake F (2007). "Cardiac autonomic imbalance in patients with reversible ventricular dysfunction takotsubo cardiomyopathy". QJM. 100 (6): 335–43. doi:10.1093/qjmed/hcm028. PMID 17483198.
- ↑ 3.0 3.1 3.2 3.3 Azzarelli S, Galassi AR, Amico F, Giacoppo M, Argentino V, Tomasello SD, Tamburino C, Fiscella A. (2006). "Clinical features of transient left ventricular apical ballooning". Am J Cardiol. 98 (9): 1273–6. PMID 17056345.
- ↑ Yamabe H, Hanaoka J, Funakoshi T; et al. (1996). "Deep negative T waves and abnormal cardiac sympathetic image (123I-MIBG) after the Great Hanshin Earthquake of 1995". Am. J. Med. Sci. 311 (5): 221–4. PMID 8615397.
- ↑ Berman M, Saute M, Porat E; et al. (2007). "Takotsubo cardiomyopathy: expanding the differential diagnosis in cardiothoracic surgery". Ann. Thorac. Surg. 83 (1): 295–8. doi:10.1016/j.athoracsur.2006.05.115. PMID 17184686.
- ↑ Akashi YJ, Sakakibara M, Miyake F (2002). "Reversible left ventricular dysfunction "takotsubo" cardiomyopathy associated with pneumothorax". Heart. 87 (2): E1. PMID 11796564.
- ↑ Wittstein IS, Thiemann DR, Lima JA; et al. (2005). "Neurohumoral features of myocardial stunning due to sudden emotional stress". N. Engl. J. Med. 352 (6): 539–48. doi:10.1056/NEJMoa043046. PMID 15703419.
- ↑ 8.0 8.1 Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE (2008). "Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning". Nat Clin Pract Cardiovasc Med. 5 (1): 22–9. doi:10.1038/ncpcardio1066. PMID 18094670.
- ↑ Mori H, Ishikawa S, Kojima S; et al. (1993). "Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli". Cardiovasc. Res. 27 (2): 192–8. PMID 8386061.
- ↑ Prasad A (2007). "Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction". Circulation. 115 (5): e56–9. doi:10.1161/CIRCULATIONAHA.106.669341. PMID 17283269.
- ↑ Elesber, AA (2007). "Four-Year Recurrence Rate and Prognosis of the Apical Ballooning Syndrome". J Amer Coll Card. 50 (5): 448–52. Unknown parameter
|month=ignored (help) - ↑ 12.0 12.1 Bybee KA, Motiei A, Syed IS, Kara T, Prasad A, Lennon RJ, Murphy JG, Hammill SC, Rihal CS, Wright RS (2006). "Electrocardiography cannot reliably differentiate transient left ventricular apical ballooning syndrome from anterior ST-segment elevation myocardial infarction". J Electrocardiol. PMID 17067626.
- ↑ Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003;41:737-742.
- ↑ San Roman Sanchez D, Medina O, Jimenez F, Rodriguez JC, Nieto V. Dynamic intraventricular obstruction in acute myocardial infarction. Echocardiography. 2001;18:515-518.
- ↑ Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539-548.
- ↑ Rivera JM, Locketz AJ, Fritz KD, et al. “Broken heart syndrome” after separation (from OxyContin). Mayo Clin Proc. 2006;81:825-828.
- ↑ Desmet WJ, Adriaenssens BF, Dens JA. Apical ballooning of the left ventricle: first series in white patients. Heart. 2003;89:1027-1031.
- ↑ Reyburn AM, Vaglio JC Jr. Transient left ventricular apical ballooning syndrome. Mayo Clin Proc. 2006;81:824.
- ↑ Ibanez B. Takotsubo Syndrome: A Bayesian Approach to Interpreting Its Pathogenesis Mayo Clin Proc. 2006; 81: 732-735
- ↑ Eitel I, von Knobelsdorff-Brekenhoff F, Bernhardt P, et al. Clinical characteristics and CV magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA 2011; 306:277-286.
- ↑ Brunetti ND, Ieva R, Rossi G, Barone N, De Gennaro L, Pellegrino PL, Mavilio G, Cuculo A, Di Biase M (2008). "Ventricular outflow tract obstruction, systolic anterior motion and acute mitral regurgitation in Tako-Tsubo syndrome". International Journal of Cardiology. 127 (3): e152–7. doi:10.1016/j.ijcard.2007.04.149. PMID 17692942. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help) - ↑ Haghi D, Röhm S, Suselbeck T, Borggrefe M, Papavassiliu T (2010). "Incidence and clinical significance of mitral regurgitation in Takotsubo cardiomyopathy". Clinical Research in Cardiology : Official Journal of the German Cardiac Society. 99 (2): 93–8. doi:10.1007/s00392-009-0078-1. PMID 19774331. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help) - ↑ http://circimaging.ahajournals.org/content/early/2011/04/15/CIRCIMAGING.110.962845.abstract
- ↑ Barkhattov TP (1991). "[The pathological preliminary period]". Felʹdsher I Akusherka (in Russian). 56 (8): 51–4. PMID 1765184. Unknown parameter
|month=ignored (help);|access-date=requires|url=(help) - ↑ Prasad A, Lerman A, Rihal CS (2008). "Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction". American Heart Journal. 155 (3): 408–17. doi:10.1016/j.ahj.2007.11.008. PMID 18294473. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help) - ↑ Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N. Kimura K. Owa M. Yoshiyama M. Miyazaki S. Haze K. Ogawa H. Honda T. Hase M. Kai R. Morii. Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol 2001;38:11-18.
- ↑ Prasad A. Apical ballooning syndrome. An important differential diagnosis of acute myocardial infarction. Circulation 2007;115:e56-59.
- ↑ Bybee KA, Prasad A, Barsness G, Wright RS, Rihal CS. Clinical Characteristics, Outcomes, and Impaired Myocardial Microcirculation in Patients with Transient Left Ventricular Apical Ballooning Syndrome: A case-series from a U.S. medial center. Am J Cardiol. 2004;94:343–346.
- ↑ Bybee KA, Kara T, Prasad A Lerman A, Barsness GW, Wright RS, Rihal CS. Transient Left Ventricular Apical Ballooning Syndrome: A mimic of ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858-865.
- ↑ Hurst RT, Askew JW, Reuss CS, Lee RW. Sweeney JP. Fortuin FD. Oh JK. Tajik AJ. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol 2006;48:579-583.
- ↑ Sharkey SW, Lesser JR, Zenovich AG, Maron MS. Lindberg J. Longe TF. Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation 2005;111:472-479.
- ↑ Elesber A, Prasad A, Bybee KA, Valeti U, Motiei A, Lerman A, Chandrasekaran K, Rihal CS. Transient Cardiac Apical Ballooning Syndrome: Prevalence and Clinical Implications of Right Ventricular Involvement. J Am Coll Cardiol 2006;47:1082-1083.
- ↑ Elesber A, Prasad A, Lennon R, Lerman A Rihal CS. Four-Year Recurrence Rate and Prognosis of the Apical Ballooning Syndrome. J Am Coll Cardiol 2007; 50:448-452.
- ↑ Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M Rihal CS, Prasad A. Myocardial Perfusion in Apical Ballooning Syndrome. Correlate of Myocardial Injury. Am Heart J 2006;152:469.e9-e13.
- ↑ Wittstein IS, Thiemann DR, Lima JA, Baughman KL. Schulman SP. Gerstenblith G. Wu KC. Rade JJ. Bivalacqua TJ. Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–548.
- ↑ Bybee KA, Kara T, Prasad A Lerman A, Barsness GW, Wright RS, Rihal CS. Transient Left Ventricular Apical Ballooning Syndrome: A mimic of ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858-865. http://www.annals.org/cgi/reprint/141/11/858.pdf
Summers MR, Lennon RJ, Prasad A. Premorbid Psychiatric and Cardiovascular Diseases in Apical Ballooning Syndrome (Takotsubo/Stress-induced Cardiomyopathy): Potential Predisposing Factors? J Am Coll Cardiol 2010;55:700-701
![Different end-systolic left ventricular (LV) silhouettes. A, [13]; B, [14]; C, [15]; D, [16]; E, [17]; and F, [18]. There is wide heterogeneity among the different patterns, varying from a relatively small akinetic apical area in C to a wide global akinesia in D and E. [19]](/images/d/d6/Takotsubo_Diagram.jpg)