Terbutaline (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
PROLONGED TOCOLYSIS
See full prescribing information for complete Boxed Warning.
Terbutaline sulfate has not been approved and should not be used for prolonged tocolysis (beyond 48 - 72 hours). In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration.
|
Overview
Terbutaline (injection) is a Beta-2 adrenergic agonist, bronchodilator and sympathomimetic that is FDA approved for the treatment of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema. There is a Black Box Warning for this drug as shown here. Common adverse reactions include palpitations, tachyarrhythmia, headache, seizure, tremor, and nervousness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Terbutaline Sulfate Injection, USP is indicated for the prevention and reversal of bronchospasm in patients 12 years of age and older with asthma and reversible bronchospasm associated with bronchitis and emphysema.
Dosage
- Ampules should be used only for subcutaneous administration and not intravenous infusion. Sterility and accurate dosing cannot be assured if the ampules are not used in accordance with DOSAGE AND ADMINISTRATION.
- The usual subcutaneous dose of Terbutaline Sulfate Injection, USP is 0.25 mg injected into the lateral deltoid area. If significant clinical improvement does not occur within 15-30 minutes, a second dose of 0.25 mg may be administered. If the patient then fails to respond within another 15-30 minutes, other therapeutic measures should be considered. The total dose within 4 hours should not exceed 0.5 mg.
Note: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terbutaline in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terbutaline (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Off-Label Guideline-Supported Use of Terbutaline in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terbutaline in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terbutaline (injection) in pediatric patients.
Contraindications
Tocolysis
- Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis.
Hypersensitivity
- Terbutaline sulfate is contraindicated in patients known to be hypersensitive to sympathomimetic amines or any components of this drug product.
Warnings
|
PROLONGED TOCOLYSIS
See full prescribing information for complete Boxed Warning.
Terbutaline sulfate has not been approved and should not be used for prolonged tocolysis (beyond 48 - 72 hours). In particular, terbutaline sulfate should not be used for maintenance tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration.
|
Deterioration of Asthma
- Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of terbutaline sulfate tablets, USP than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and the treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
Use of Anti-Inflammatory Agents
- The use of beta-adrenergic agonist bronchodilators alone may not be adequate to control asthma in many patients. Early consideration should be given to adding anti-inflammatory agents, e.g., corticosteroids.
Cardiovascular Effects
- Terbutaline sulfate tablets, USP, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of terbutaline sulfate tablets, USP at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, terbutaline sulfate tablets, USP, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Seizures
- There have been rare reports of seizures in patients receiving terbutaline; seizures did not recur in these patients after the drug was discontinued.
Precautions
(Terbutaline, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, including ischemic heart disease, hypertension, and cardiac arrhythmias; in patients with hyperthyroidism or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines or who have convulsive disorders. Significant changes in systolic and diastolic blood pressure have been seen and could be expected to occur in some patients after use of any beta-adrenergic bronchodilator.
- Immediate hypersensitivity reactions and exacerbations of bronchospasm have been reported after terbutaline administration.
- Beta-adrenergic agonist medications may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
- Large doses of intravenous terbutaline have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions observed with Terbutaline Sulfate Injection, USP are similar to those commonly seen with other sympathomimetic agents. All these reactions are transient in nature and usually do not require treatment.
- The following table compares adverse reactions seen in patients treated with terbutaline sulfate Injection (0.25 mg and 0.5 mg), with those seen in patients treated with epinephrine injection (0.25 mg and 0.5 mg), during eight double-blind crossover studies involving a total of 214 patients.

Postmarketing Experience
There is limited information regarding Terbutaline (injection) Postmarketing Experience in the drug label.
Drug Interactions
Drug Interactions
- The concomitant use of terbutaline sulfate tablets, USP with other sympathomimetic agents is not recommended, since the combined effect on the cardiovascular system may be deleterious to the patient. However, this does not preclude the use of an aerosol bronchodilator of the adrenergic-stimulant type for the relief of an acute bronchospasm in patients receiving chronic oral therapy with terbutaline sulfate tablets, USP.
Monoamine Oxidase Inhibitors and Tricyclic Antidepressants
- Terbutaline sulfate should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, since the action of terbutaline sulfate on the vascular system may be potentiated.
Beta-Blockers
- Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-agonists, such as terbutaline sulfate tablets, USP, but may produce severe bronchospasm in asthmatic patients. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic blocking agents in patients with asthma. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
Diuretics
- The ECG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop diuretics or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of beta-agonists with non-potassium sparing diuretics.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of terbutaline sulfate in pregnant women. Published animal studies show that rat offspring exhibit alterations in behavior and brain development, including decreased cellular proliferation and differentiation when dams were treated subcutaneously with terbutaline during the late stage of pregnancy and lactation period. Terbutaline exposures in rat dams were approximately 6.5 times the common human dose in adults of 15 mg/day, on a mg/m2 basis.
- Oral terbutaline sulfate has not been approved and should not be used for acute or maintenance tocolysis. In particular, terbutaline sulfate should not be used for tocolysis in the outpatient or home setting. Serious adverse reactions, including death, have been reported after administration of terbutaline sulfate to pregnant women. In the mother, these adverse reactions include increased heart rate, transient hyperglycemia, hypokalemia, cardiac arrhythmias, pulmonary edema and myocardial ischemia. Increased fetal heart rate and neonatal hypoglycemia may occur as a result of maternal administration.
- In animal embryofetal development studies, no teratogenic effects were observed in offspring when pregnant rats and rabbits received terbutaline sulfate at oral doses up to 50 mg/kg/day, approximately 32 and 65 times, respectively, the maximum recommended daily oral dose for adults, on a mg/m2 basis.
- Terbutaline sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Terbutaline (injection) in women who are pregnant.
Labor and Delivery
- Because of the potential for beta-agonist interference with uterine contractility, use of Terbutaline Sulfate Injection, USP for relief of bronchospasm during labor should be restricted to those patients in whom the benefits clearly outweigh the risk. Terbutaline crosses the placenta. After single dose IV administration of terbutaline to 22 women in late pregnancy who were delivered by elective Cesarean section due to clinical reasons, umbilical blood levels of terbutaline were found to range from 11% to 48% of the maternal blood levels.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Therefore, Terbutaline Sulfate Injection, USP should be used during nursing only if the potential benefit justifies the possible risk to the newborn.
Pediatric Use
- Terbutaline sulfate tablets, USP are not recommended for patients under the age of 12 years because of insufficient clinical data to establish safety and effectiveness.
Geriatic Use
- Clinical Studies of terbutaline sulfate injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Terbutaline (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Terbutaline (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Terbutaline (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Terbutaline (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Terbutaline (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Terbutaline (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Terbutaline (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Terbutaline (injection) and IV administrations.
Overdosage
- The median subcutaneous lethal dose of terbutaline sulfate in mature rats is approximately 165 mg/kg (approximately 90 times the maximum recommended daily oral dose for adults on a mg/m2 basis). The median subcutaneous lethal dose of terbutaline sulfate in young rats is approximately 2000 mg/kg (approximately 1100 times the maximum recommended daily oral dose for adults on a mg/m2 basis).
- The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms listed under adverse reactions, e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats per minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, and insomnia. Hypokalemia may also occur.
- There is no specific antidote. Treatment consists of discontinuation of terbutaline sulfate tablets, USP together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of terbutaline sulfate tablets.
- In the alert patient who has taken excessive oral medication, the stomach should be emptied by induced emesis followed by lavage. In the unconscious patient, the airway should be secured with a cuffed endotracheal tube before lavage, and emesis should not be induced. Instillation of activated charcoal slurry may help reduce absorption of terbutaline. Adequate respiratory exchange should be maintained, and cardiac and respiratory support provided as needed. The patient should be monitored until signs and symptoms of overdosage have subsided.
Pharmacology
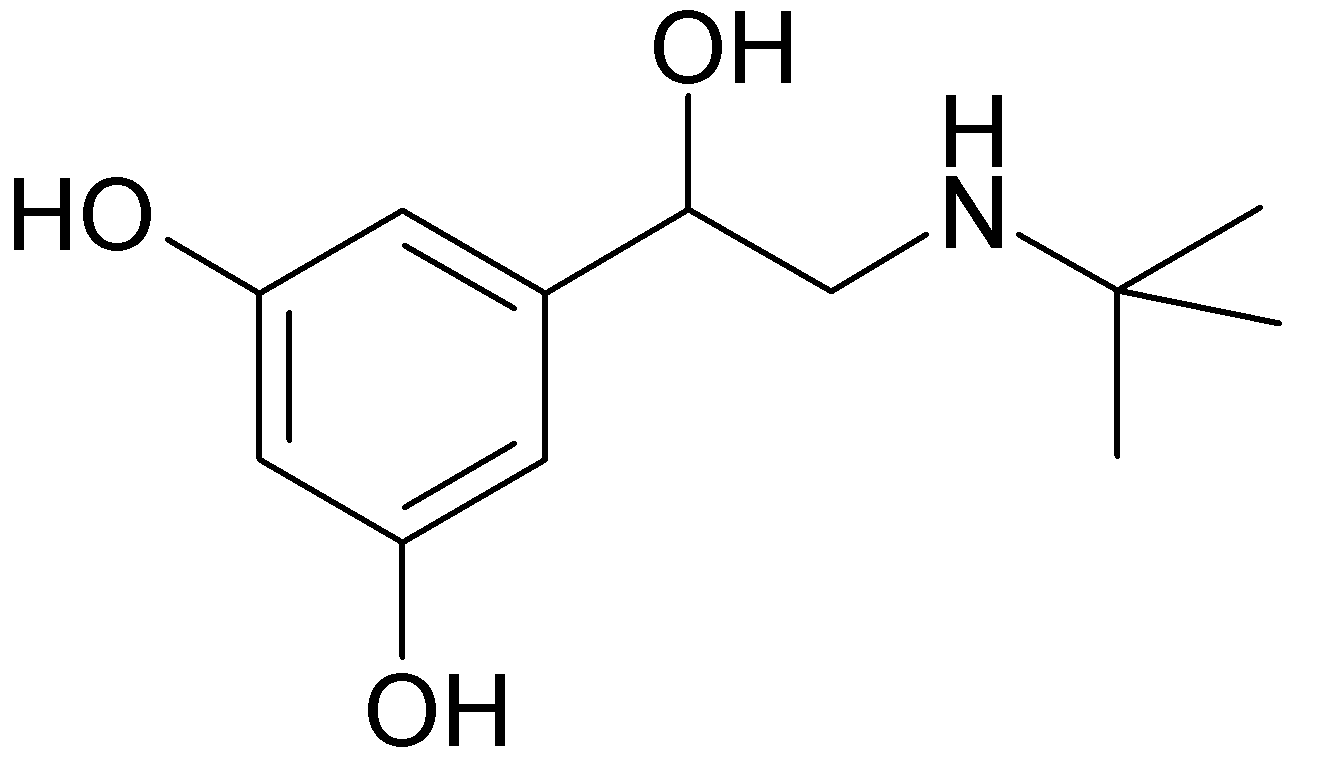
| |
| |
1 : 1 mixture (racemate)Terbutaline
| |
| Systematic (IUPAC) name | |
| (RS)-5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diol | |
| Identifiers | |
| CAS number | |
| ATC code | R03 R03CC03 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 225.284 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 25% |
| Metabolism | GI tract (oral), liver; CYP450: unknown |
| Half life | 11-16 hours |
| Excretion | urine 90% (60% unchanged), bile/faeces |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status | |
| Routes | SQ, Oral, Inhaled |
Mechanism of Action
- Terbutaline Sulfate Injection, USP is a beta-adrenergic receptor agonist. In vitro and in vivopharmacologic studies have demonstrated that terbutaline exerts a preferential effect on beta2-adrenergic receptors. While it is recognized that beta2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta2-receptors in the human heart, existing in a concentration between 10%-50%. The precise function of these receptors has not been established . Controlled clinical studies in patients given terbutaline subcutaneously have not revealed a preferential beta2-adrenergic effect.
- The pharmacologic effects of beta-adrenergic agonists, including terbutaline, are at least in part attributable to stimulation through beta-adrenergic receptors of intracellular adenyl cyclase, the enzyme which catalyzes the conversion of adenosine triphosphate (ATP) to cyclic 3', 5'-adenosine monophosphate (cAMP). Increased cAMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
- Controlled clinical studies have shown that Terbutaline Sulfate Injection, USP relieves bronchospasm in acute and chronic obstructive pulmonary disease by significantly increasing pulmonary flow rates (e.g., an increase of 15% or more in FEV1). After subcutaneous administration of 0.25 mg of Terbutaline Sulfate Injection, USP, a measurable change in expiratory flow rate usually occurs within 5 minutes, and a clinically significant increase in FEV1 occurs within 15 minutes. The maximum effect usually occurs within 30-60 minutes, and clinically significant bronchodilator activity may continue for 1.5 to 4 hours. The duration of clinically significant improvement is comparable to that observed with equimilligram doses of epinephrine.
Structure
- Terbutaline sulfate USP, the active ingredient, is a beta-adrenergic agonist bronchodilator available as a sterile, nonpyrogenic, aqueous solution in ampules, for subcutaneous administration. Each milliliter of solution contains 1 mg of terbutaline sulfate USP (0.82 mg of the free base); sodium chloride USP, for isotonicity; hydrochloric acid NF, for adjustment to a target pH of 4; and water for injection.
- Terbutaline sulfate is (±)-α-[(tert-butylamino) methyl]-3,5-dihydroxybenzyl alcohol sulfate (2:1) (salt). The empirical formula is (C12H19NO3)2 • H2SO4 and the structural formula is:

- Terbutaline sulfate USP is a white to gray-white crystalline powder. It is odorless or has a faint odor of acetic acid. It is soluble in water and in 0.1 N hydrochloric acid, slightly soluble in methanol, and insoluble in chloroform. Its molecular weight is 548.65.
Pharmacodynamics
- Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histological evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical significance of these findings is unknown.
Pharmacokinetics
- Subcutaneous administration of 0.5 mg of terbutaline sulfate to 17 healthy, adult, male subjects resulted in mean (SD) peak plasma terbutaline concentrations of 9.6 (3.6) ng/mL, which was observed at a median (range) time of 0.5 (0.08-1.0) hours after dosing. The mean (SD) AUC (0-48) and total body clearance values were 29.4 (14.2) hr•ng/mL, and 311 (112) mL/min respectively. The terminal half-life was determined in 9 of the 17 subjects and had a mean (SD) of 5.7 (2.0) hours.
- After subcutaneous administration of 0.25 mg of terbutaline sulfate to two male subjects, peak terbutaline serum concentrations of 5.2 and 5.3 ng/mL were observed at about 20 minutes after dosing.
- Elimination half-life of the drug in 10 of 14 patients was approximately 2.9 hours after subcutaneous administration, but longer elimination half-lives (between 6-14 hours) were found in the other 4 patients. About 90% of the drug was excreted in the urine at 96 hours after subcutaneous administration, with about 60% of this being unchanged drug. It appears that the sulfate conjugate is a major metabolite of terbutaline and urinary excretion is the primary route of elimination.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a 2-year study in Sprague-Dawley rats, terbutaline sulfate caused a significant and dose-related increase in the incidence of benign leiomyomas of the mesovarium at dietary doses of 50 mg/kg and above (approximately 810 times the maximum recommended daily subcutaneous (sc) dose for adults on a mg/m2 basis). In a 21 month study in CD-1 mice, terbutaline sulfate showed no evidence of tumorigenicity at dietary doses of up to 200 mg/kg (approximately 1,600 times the maximum recommended daily sc dose for adults on a mg/m2 basis). The mutagenicity potential of terbutaline sulfate has not been determined.
- Reproductive studies in rats using terbutaline sulfate demonstrated no impairment of fertility at oral doses up to 50 mg/kg (approximately 810 times the maximum recommended daily sc dose for adults on a mg/m2 basis.)
Clinical Studies
There is limited information regarding Terbutaline (injection) Clinical Studies in the drug label.
How Supplied
- Ampules 1 mg/mL – The drug is supplied at a volume of 1 mL contained in a 2 mL clear glass ampule. Each ampule contains 1 mg of terbutaline sulfate per 1 mL of solution; 0.25 mL of solution will provide the clinical dose of 0.25 mg.
- Ampules are expiration-dated
- Discard unused portion after single patient use.
- Terbutaline Sulfate Injection, USP, 1 mg/mL is available as a 1 mL ampule in packs of 10.
- NDC 17478-933-01
- Store at 20° to 25° C (68º to 77°F). [See USP Controlled Room Temperature].
- Protect from light by storing ampules in original carton until dispensed.
- Do not use if solution is discolored.
Storage
- Store at controlled room temperature 15° to 30°C (59° to 86°F). Dispense in tightly-closed, light-resistant container (USP).
Images
Drug Images
{{#ask: Page Name::Terbutaline (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Terbutaline (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Terbutaline (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Terbutaline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- TERBUTALINE SULFATE
Look-Alike Drug Names
There is limited information regarding Terbutaline (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Terbutaline (injection) |Label Name=Terbutaline03.png
}}
{{#subobject:
|Label Page=Terbutaline (injection) |Label Name=Terbutaline04.png
}}
{{#subobject:
|Label Page=Terbutaline (injection) |Label Name=Terbutaline05.png
}}