Ceftibuten clinical pharmacology: Difference between revisions
(Created page with "__NOTOC__ {{Ceftibuten}} {{CMG}};{{AE}}{{AK}} ===PHARMACOKINETICS=== '''Absorption''' CEDAX CAPSULES Ceftibuten is rapidly absorbed after oral administration of CEDAX Ca...") |
No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Ceftibuten}} | {{Ceftibuten}} | ||
{{CMG}};{{AE}}{{AK}} | {{CMG}};{{AE}}{{AK}} | ||
| Line 16: | Line 14: | ||
Ceftibuten is rapidly absorbed after oral administration of CEDAX Oral Suspension. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 9-mg/kg dose of CEDAX Oral Suspension to 32 fasting pediatric patients (6 months to 12 years of age) are displayed in the following table: | Ceftibuten is rapidly absorbed after oral administration of CEDAX Oral Suspension. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 9-mg/kg dose of CEDAX Oral Suspension to 32 fasting pediatric patients (6 months to 12 years of age) are displayed in the following table: | ||
[[image:Cefti1.png]] | |||
The absolute bioavailability of CEDAX Oral Suspension has not been determined. The plasma concentrations of ceftibuten in pediatric patients are dose proportional following single doses of CEDAX Capsules of 200 mg and 400 mg and of CEDAX Oral Suspension between 4.5 mg/kg and 9 mg/kg. | The absolute bioavailability of CEDAX Oral Suspension has not been determined. The plasma concentrations of ceftibuten in pediatric patients are dose proportional following single doses of CEDAX Capsules of 200 mg and 400 mg and of CEDAX Oral Suspension between 4.5 mg/kg and 9 mg/kg. | ||
Distribution | '''Distribution''' | ||
CEDAX CAPSULES | CEDAX CAPSULES | ||
| Line 34: | Line 32: | ||
Ceftibuten is 65% bound to plasma proteins. The protein binding is independent of plasma ceftibuten concentration. | Ceftibuten is 65% bound to plasma proteins. The protein binding is independent of plasma ceftibuten concentration. | ||
Tissue Penetration | '''Tissue Penetration''' | ||
Bronchial secretions | Bronchial secretions | ||
| Line 40: | Line 38: | ||
In a study of 15 adults administered a single 400-mg dose of ceftibuten and scheduled to undergo bronchoscopy, the mean concentrations in epithelial lining fluid and bronchial mucosa were 15% and 37%, respectively, of the plasma concentrations. | In a study of 15 adults administered a single 400-mg dose of ceftibuten and scheduled to undergo bronchoscopy, the mean concentrations in epithelial lining fluid and bronchial mucosa were 15% and 37%, respectively, of the plasma concentrations. | ||
Sputum | '''Sputum''' | ||
Ceftibuten sputum levels average approximately 7% of the concomitant plasma ceftibuten level. In a study of 24 adults administered ceftibuten 200 mg bid or 400 mg qd, the average Cmax in sputum (1.5 µg/mL) occurred at 2 hours postdose and the average Cmax in plasma (17 µg/mL) occurred at 2 hours postdose. | Ceftibuten sputum levels average approximately 7% of the concomitant plasma ceftibuten level. In a study of 24 adults administered ceftibuten 200 mg bid or 400 mg qd, the average Cmax in sputum (1.5 µg/mL) occurred at 2 hours postdose and the average Cmax in plasma (17 µg/mL) occurred at 2 hours postdose. | ||
Middle-ear fluid (MEF) | '''Middle-ear fluid (MEF)''' | ||
In a study of 12 pediatric patients administered 9 mg/kg, ceftibuten MEF area under the curve (AUC) averaged approximately 70% of the plasma AUC. In the same study, Cmax values were 14.3 ± 2.7 µg/mL in MEF at 4 hours postdose and 14.5 ± 3.7 µg/mL in plasma at 2 hours postdose. | In a study of 12 pediatric patients administered 9 mg/kg, ceftibuten MEF area under the curve (AUC) averaged approximately 70% of the plasma AUC. In the same study, Cmax values were 14.3 ± 2.7 µg/mL in MEF at 4 hours postdose and 14.5 ± 3.7 µg/mL in plasma at 2 hours postdose. | ||
Tonsillar tissue | '''Tonsillar tissue''' | ||
Data on ceftibuten penetration into tonsillar tissue are not available. | Data on ceftibuten penetration into tonsillar tissue are not available. | ||
| Line 56: | Line 54: | ||
Data on ceftibuten penetration into cerebrospinal fluid are not available. | Data on ceftibuten penetration into cerebrospinal fluid are not available. | ||
Metabolism and Excretion | ===Metabolism and Excretion=== | ||
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer. The trans-isomer is approximately ⅛ as antimicrobially potent as the cis-isomer. | A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer. The trans-isomer is approximately ⅛ as antimicrobially potent as the cis-isomer. | ||
| Line 62: | Line 60: | ||
Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces. In 6 healthy adult male volunteers, approximately 56% of the administered dose of ceftibuten was recovered from urine and 39% from the feces within 24 hours. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment (see DOSAGE AND ADMINISTRATION). | Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces. In 6 healthy adult male volunteers, approximately 56% of the administered dose of ceftibuten was recovered from urine and 39% from the feces within 24 hours. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment (see DOSAGE AND ADMINISTRATION). | ||
Food Effect on Absorption | '''Food Effect on Absorption''' | ||
Food affects the bioavailability of ceftibuten from CEDAX Capsules and CEDAX Oral Suspension. | Food affects the bioavailability of ceftibuten from CEDAX Capsules and CEDAX Oral Suspension. | ||
| Line 70: | Line 68: | ||
The effect of food on the bioavailability of CEDAX Oral Suspension was evaluated in 18 healthy adult male volunteers who ingested 400 mg of CEDAX Oral Suspension after an overnight fast or immediately after a standardized breakfast. Results obtained demonstrated a decrease in Cmax of 26% and an AUC of 17% when CEDAX Oral Suspension was administered with a high-fat breakfast, and a decrease in Cmax of 17% and an AUC of 12% when CEDAX Oral Suspension was administered with a low-calorie nonfat breakfast (see PRECAUTIONS). | The effect of food on the bioavailability of CEDAX Oral Suspension was evaluated in 18 healthy adult male volunteers who ingested 400 mg of CEDAX Oral Suspension after an overnight fast or immediately after a standardized breakfast. Results obtained demonstrated a decrease in Cmax of 26% and an AUC of 17% when CEDAX Oral Suspension was administered with a high-fat breakfast, and a decrease in Cmax of 17% and an AUC of 12% when CEDAX Oral Suspension was administered with a low-calorie nonfat breakfast (see PRECAUTIONS). | ||
Bioequivalence of Dosage Formulations | '''Bioequivalence of Dosage Formulations''' | ||
A study in 18 healthy adult male volunteers demonstrated that a 400-mg dose of CEDAX Capsules produced equivalent concentrations to a 400-mg dose of CEDAX Oral Suspension. Average Cmax values were 15.6 (3.1) µg/mL for the capsule and 17.0 (3.2) µg/mL for the suspension. Average AUC values were 80.1 (14.4) µg∙hr/mL for the capsule and 87.0 (12.2) µg∙hr/mL for the suspension. | A study in 18 healthy adult male volunteers demonstrated that a 400-mg dose of CEDAX Capsules produced equivalent concentrations to a 400-mg dose of CEDAX Oral Suspension. Average Cmax values were 15.6 (3.1) µg/mL for the capsule and 17.0 (3.2) µg/mL for the suspension. Average AUC values were 80.1 (14.4) µg∙hr/mL for the capsule and 87.0 (12.2) µg∙hr/mL for the suspension. | ||
Special Populations | '''Special Populations''' | ||
Geriatric patients | Geriatric patients | ||
Revision as of 21:01, 30 December 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
PHARMACOKINETICS
Absorption
CEDAX CAPSULES
Ceftibuten is rapidly absorbed after oral administration of CEDAX Capsules. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 400-mg dose of CEDAX Capsules to 12 healthy adult male volunteers (20 to 39 years of age) are displayed in the table below. When CEDAX Capsules were administered once daily for 7 days, the average Cmax was 17.9 µg/mL on day 7. Therefore, ceftibuten accumulation in plasma is about 20% at steady state.
CEDAX ORAL SUSPENSION
Ceftibuten is rapidly absorbed after oral administration of CEDAX Oral Suspension. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 9-mg/kg dose of CEDAX Oral Suspension to 32 fasting pediatric patients (6 months to 12 years of age) are displayed in the following table:
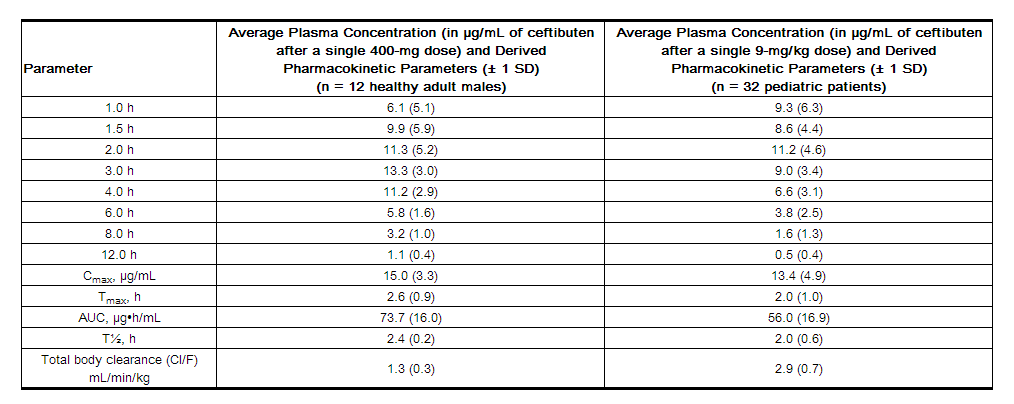
The absolute bioavailability of CEDAX Oral Suspension has not been determined. The plasma concentrations of ceftibuten in pediatric patients are dose proportional following single doses of CEDAX Capsules of 200 mg and 400 mg and of CEDAX Oral Suspension between 4.5 mg/kg and 9 mg/kg.
Distribution
CEDAX CAPSULES
The average apparent volume of distribution (V/F) of ceftibuten in 6 adult subjects is 0.21 L/kg (± 1 SD = 0.03 L/kg).
CEDAX ORAL SUSPENSION
The average apparent volume of distribution (V/F) of ceftibuten in 32 fasting pediatric patients is 0.5 L/kg (± 1 SD = 0.2 L/kg).
Protein Binding
Ceftibuten is 65% bound to plasma proteins. The protein binding is independent of plasma ceftibuten concentration.
Tissue Penetration
Bronchial secretions
In a study of 15 adults administered a single 400-mg dose of ceftibuten and scheduled to undergo bronchoscopy, the mean concentrations in epithelial lining fluid and bronchial mucosa were 15% and 37%, respectively, of the plasma concentrations.
Sputum
Ceftibuten sputum levels average approximately 7% of the concomitant plasma ceftibuten level. In a study of 24 adults administered ceftibuten 200 mg bid or 400 mg qd, the average Cmax in sputum (1.5 µg/mL) occurred at 2 hours postdose and the average Cmax in plasma (17 µg/mL) occurred at 2 hours postdose.
Middle-ear fluid (MEF)
In a study of 12 pediatric patients administered 9 mg/kg, ceftibuten MEF area under the curve (AUC) averaged approximately 70% of the plasma AUC. In the same study, Cmax values were 14.3 ± 2.7 µg/mL in MEF at 4 hours postdose and 14.5 ± 3.7 µg/mL in plasma at 2 hours postdose.
Tonsillar tissue
Data on ceftibuten penetration into tonsillar tissue are not available.
Cerebrospinal fluid
Data on ceftibuten penetration into cerebrospinal fluid are not available.
Metabolism and Excretion
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer. The trans-isomer is approximately ⅛ as antimicrobially potent as the cis-isomer.
Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces. In 6 healthy adult male volunteers, approximately 56% of the administered dose of ceftibuten was recovered from urine and 39% from the feces within 24 hours. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment (see DOSAGE AND ADMINISTRATION).
Food Effect on Absorption
Food affects the bioavailability of ceftibuten from CEDAX Capsules and CEDAX Oral Suspension.
The effect of food on the bioavailability of CEDAX Capsules was evaluated in 26 healthy adult male volunteers who ingested 400 mg of CEDAX Capsules after an overnight fast or immediately after a standardized breakfast. Results showed that food delays the time of Cmax by 1.75 hours, decreases the Cmaxby 18%, and decreases the extent of absorption (AUC) by 8%.
The effect of food on the bioavailability of CEDAX Oral Suspension was evaluated in 18 healthy adult male volunteers who ingested 400 mg of CEDAX Oral Suspension after an overnight fast or immediately after a standardized breakfast. Results obtained demonstrated a decrease in Cmax of 26% and an AUC of 17% when CEDAX Oral Suspension was administered with a high-fat breakfast, and a decrease in Cmax of 17% and an AUC of 12% when CEDAX Oral Suspension was administered with a low-calorie nonfat breakfast (see PRECAUTIONS).
Bioequivalence of Dosage Formulations
A study in 18 healthy adult male volunteers demonstrated that a 400-mg dose of CEDAX Capsules produced equivalent concentrations to a 400-mg dose of CEDAX Oral Suspension. Average Cmax values were 15.6 (3.1) µg/mL for the capsule and 17.0 (3.2) µg/mL for the suspension. Average AUC values were 80.1 (14.4) µg∙hr/mL for the capsule and 87.0 (12.2) µg∙hr/mL for the suspension.
Special Populations Geriatric patients
Ceftibuten pharmacokinetics have been investigated in elderly (65 years of age and older) men (n = 8) and women (n = 4). Each volunteer received ceftibuten 200-mg capsules twice daily for 3½ days. The average Cmax was 17.5 (3.7) µg/mL after 3½ days of dosing compared to 12.9 (2.1) µg/mL after the first dose; ceftibuten accumulation in plasma was 40% at steady state. Information regarding the renal function of these volunteers was not available; therefore, the significance of this finding for clinical use of CEDAX Capsules in elderly patients is not clear. Ceftibuten dosage adjustment in elderly patients may be necessary (see DOSAGE AND ADMINISTRATION).
Patients with renal insufficiency
Ceftibuten pharmacokinetics have been investigated in adult patients with renal dysfunction. The ceftibuten plasma half-life increased and apparent total clearance (CI/F) decreased proportionately with increasing degree of renal dysfunction. In 6 patients with moderate renal dysfunction (creatinine clearance 30 to 49 mL/min), the plasma half-life of ceftibuten increased to 7.1 hours and CI/F decreased to 30 mL/min. In 6 patients with severe renal dysfunction (creatinine clearance 5 to 29 mL/min), the half-life increased to 13.4 hours and CI/F decreased to 16 mL/min. In 6 functionally anephric patients (creatinine clearance <5 mL/min), the half-life increased to 22.3 hours and CI/F decreased to 11 mL/min (a 7- to 8-fold change compared to healthy volunteers). Hemodialysis removed 65% of the drug from the blood in 2 to 4 hours. These changes serve as the basis for dosage adjustment recommendations in adult patients with mild to severe renal dysfunction (see DOSAGE AND ADMINISTRATION).
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050686s016lbl.pdf