Pimobendane: Difference between revisions
No edit summary |
No edit summary |
||
| Line 84: | Line 84: | ||
==Synthesis== | ==Synthesis== | ||
Pimobendan can be synthesized beginning with [[anisoyl chloride]]. | Pimobendan can be synthesized beginning with [[anisoyl chloride]]. | ||
[[File:Pimobendan synthesis.png|400px | [[File:Pimobendan synthesis.png|400px|left|none]] | ||
==See also== | ==See also== | ||
Revision as of 21:08, 28 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60 to 65% |
| Elimination half-life | 0.4 hours |
| Excretion | In feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H18N4O2 |
| Molar mass | 334.37 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Pimobendane |
|
Articles |
|---|
|
Most recent articles on Pimobendane Most cited articles on Pimobendane |
|
Media |
|
Powerpoint slides on Pimobendane |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Pimobendane at Clinical Trials.gov Clinical Trials on Pimobendane at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Pimobendane
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Pimobendane Discussion groups on Pimobendane Patient Handouts on Pimobendane Directions to Hospitals Treating Pimobendane Risk calculators and risk factors for Pimobendane
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Pimobendane |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Pimobendan (or pimobendane)(INN) is a veterinary medication manufactured by Boehringer Ingelheim under the trade names Vetmedin and Acardi (Japan). It is both a calcium sensitizer and a selective inhibitor of phosphodiesterase III (PDE3) with positive inotropic and vasodilator effects.
Pimobendan is used in the management of heart failure in dogs, most commonly caused by myxomatous mitral valve disease (also previously known as endocardiosis), or dilated cardiomyopathy.[1] Research has shown that pimobendan increases survival time and improves quality of life in canine patients with congestive heart failure secondary to mitral valve disease when compared with benazepril, an angiotensin-converting-enzyme (ACE) inhibitor.[2] Under the trade name Acardi, it is available for human use in Japan.[3]
Mechanism of action
Pimobendan is a positive inotrope (increases myocardial contractility). It sensitizes and increases the binding efficiency of cardiac troponin in the myofibril to the calcium ions that are already present in systole. In normal hearts it increases the consumption of oxygen and energy to the same degree as dobutamine but in diseased hearts it may not.[4] [5] Pimobendan also causes peripheral vasodilation by inhibiting the function of phosphodiesterase III. This results in decreased resistance to blood flow through systemic arterioles, which decreases afterload (decreases the failing heart's workload) and reduces the amount of mitral regurgitation.[6][7]
Pharmacokinetics
Pimobendan is absorbed rapidly when given via the oral route and has a bioavailability of 60-65%.[8] Bioavailability is markedly decreased when ingested with food. It is metabolized into an active metabolite (desmethylpimobendan) by the liver. The parent compound, pimobendan, is a potent calcium sensitizer while desmethylpimobendan is a more potent phosphodiesterase III inhibitor.[9] The half-life of pimobendan in the blood is 0.4 hours and the half-life of its metabolite is 2 hours. Elimination is by excretion in the bile and then feces. Pimobendan is 90–95% bound to plasma proteins in circulation. This may have implications in patients suffering from low blood protein levels (hypoproteinemia/hypoalbuminemia) and in patients that are on concurrent therapies that are also highly protein bound.
Combinations
Pimobendan is often used in combination with three other drugs to palliate dogs with heart failure (pulmonary edema, pleural effusion, ascites). These are:
- Furosemide, a diuretic, to reduce edema and effusion.
- Spironolactone, an aldosterone antagonist. This has two actions, firstly, as a potassium-sparing diuretic, although its diuretic properties are small compared with those of furosemide. Secondly, it reduces aldosterone-mediated myocardial fibrosis, possibly slowing the progression of heart disease.
- An ACE inhibitor, often enalapril (trade name Enacard) or benazepril (Fortekor). These drugs inhibit the action of angiotensin-converting enzyme, producing a balanced vasodilation, along with other potentially favorable effects.
Other drugs may also be used as required to manage certain arrhythmias that are often associated with heart disease.
Synthesis
Pimobendan can be synthesized beginning with anisoyl chloride.
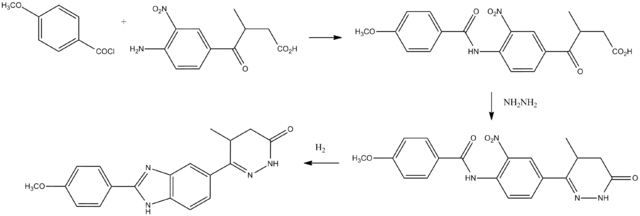
See also
References
- ↑ Gordon SG, Miller MW, Saunders AB (2006). "Pimobendan in heart failure therapy—a silver bullet?". J Am Anim Hosp Assoc. 42 (2): 90–3. PMID 16527909.
- ↑ Häggström J, Boswood A, O'Grady M; et al. (July 2008). "Effect of Pimobendan or Benazepril Hydrochloride on Survival Times in Dogs with Congestive Heart Failure Caused by Naturally Occurring Myxomatous Mitral Valve Disease: The QUEST Study". J. Vet. Intern. Med. 22 (5): 1124–35. doi:10.1111/j.1939-1676.2008.0150.x. PMID 18638016.
- ↑ "Kusuri-no-Shiori Drug Information Sheet". RAD-AR Council, Japan. April 2005. Retrieved 2008-08-06.
- ↑ Hata K1, Goto Y, Futaki S, Ohgoshi Y, Yaku H, Kawaguchi O, Takasago T, Saeki A, Taylor TW, Nishioka T, et al. Mechanoenergetic effects of pimobendan in canine left ventricles. Comparison with dobutamine. Circulation. 1992 Oct;86(4):1291-301.
- ↑ Goto Y1, Hata K. Mechanoenergetic effect of pimobendan in failing dog hearts. Heart Vessels. 1997;Suppl 12:103-5.
- ↑ Verdouw PD, Hartog JM, Duncker DJ, Roth W, Saxena PR. Cardiovascular profile of pimobendan, a benzimidazole-pyridazinone derivative with vasodilating and inotropic properties. Eur J Pharmacol. 1986 Jul 15;126(1-2):21-30.
- ↑ Kanno N, Kuse H, Kawasaki M, Hara A, Kano R, Sasaki Y. Effects of pimobendan for mitral valve regurgitation in dogs. J Vet Med Sci. 2007 Apr;69(4):373-7.
- ↑ http://www.vetmedin.com/Vetmedin%20Insert_6-07.pdf
- ↑ Hanzlicek AS1, Gehring R, Kukanich B, Kukanich KS, Borgarelli M, Smee N, Olson EE, Margiocco M. Pharmacokinetics of oral pimobendan in healthy cats. J Vet Cardiol. 2012 Dec;14(4):489-96.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.
- Pages using duplicate arguments in template calls
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Benzimidazoles
- Lactams
- Inotropic agents
- Vasodilators
- Pyridazines
- Phenol ethers
- Drug