Cefdinir description: Difference between revisions
No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Cefdinir}} | {{Cefdinir}} | ||
{{CMG}} | {{CMG}}; {{AE}} {{SS}} | ||
==Description== | |||
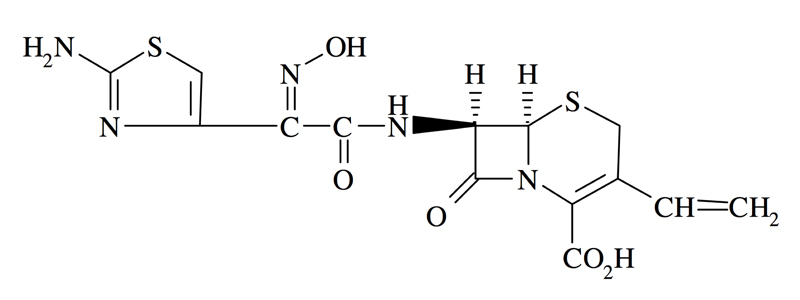
Cefdinir capsules contains the active ingredient cefdinir, an extended-spectrum, semisynthetic [[cephalosporin]], for oral administration. Chemically, cefdinir is [6R-[6α,7β(Z)]]-7-[[(2-amino-4 thiazolyl) (hydroxyimino) acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Cefdinir is a white to slightly brownish-yellow solid. It is slightly soluble in dilute hydrochloric acid and sparingly soluble in 0.1 M pH 7.0 phosphate buffer. The molecular formula is C14H13N5O5S2 and the molecular weight is 395.42. Cefdinir has the structural formula shown below: | |||
{| | {| | ||
|- | |- | ||
|[[File:Cefedinir 1.jpg|thumb| | |[[File:Cefedinir 1 1.jpg|thumb|800px|left]] | ||
|- | |- | ||
|} | |} | ||
Cefdinir capsules contain 300 mg of cefdinir and the following inactive ingredients: | Cefdinir capsules contain 300 mg of cefdinir and the following inactive ingredients: [[carboxymethyl cellulose]] calcium; colloidal silicon dioxide; and magnesium stearate. The capsule shells contain gelatin and [[titanium]] dioxide.<ref>{{Cite web | last = |first = |title = http://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50739S2LBL.pdf |url =http://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50739S2LBL.pdf |publisher = |date = | accessdate = }}</ref> | ||
==References== | |||
{{Reflist}} | |||
{{FDA}} | |||
[[Category:Antibiotics]] | |||
[[Category:Wikinfect]] | |||
Latest revision as of 00:49, 6 January 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Description
Cefdinir capsules contains the active ingredient cefdinir, an extended-spectrum, semisynthetic cephalosporin, for oral administration. Chemically, cefdinir is [6R-[6α,7β(Z)]]-7-[[(2-amino-4 thiazolyl) (hydroxyimino) acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid. Cefdinir is a white to slightly brownish-yellow solid. It is slightly soluble in dilute hydrochloric acid and sparingly soluble in 0.1 M pH 7.0 phosphate buffer. The molecular formula is C14H13N5O5S2 and the molecular weight is 395.42. Cefdinir has the structural formula shown below:
 |
Cefdinir capsules contain 300 mg of cefdinir and the following inactive ingredients: carboxymethyl cellulose calcium; colloidal silicon dioxide; and magnesium stearate. The capsule shells contain gelatin and titanium dioxide.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50739S2LBL.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.