Cefdinir clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Studies
Community-Acquired Bacterial Pneumonia
In a controlled, double-blind study in adults and adolescents conducted in the U.S., cefdinir BID was compared with cefaclor 500 mg TID. Using strict evaluability and microbiologic/clinical response criteria 6 to 14 days posttherapy, the following clinical cure rates, presumptive microbiologic eradication rates, and statistical outcomes were obtained:
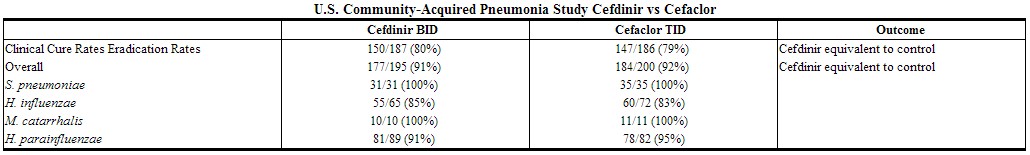 |
In a second controlled, investigator-blind study in adults and adolescents conducted primarily in Europe, cefdinir BID was compared with amoxicillin/clavulanate 500/125 mg TID. Using strict evaluability and clinical response criteria 6 to 14 days posttherapy, the following clinical cure rates, presumptive microbiologic eradication rates, and statistical outcomes were obtained:
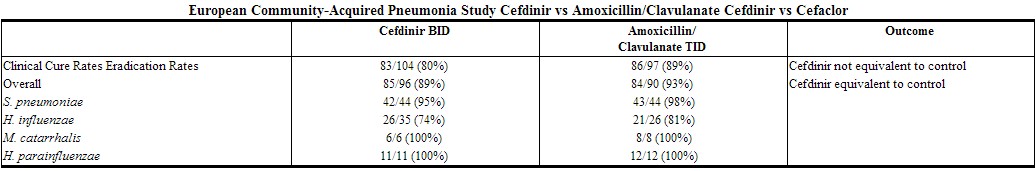 |
Streptococcal Pharyngitis/Tonsillitis
In four controlled studies conducted in the U.S., cefdinir was compared with 10 days of penicillin in adult, adolescent, and pediatric patients. Two studies (one in adults and adolescents, the other in pediatric patients) compared 10 days of cefdinir QD or BID to penicillin 250 mg or 10 mg/kg QID. Using strict evaluability and microbiologic/clinical response criteria 5 to 10 days posttherapy, the following clinical cure rates, microbiologic eradication rates, and statistical outcomes were obtained:
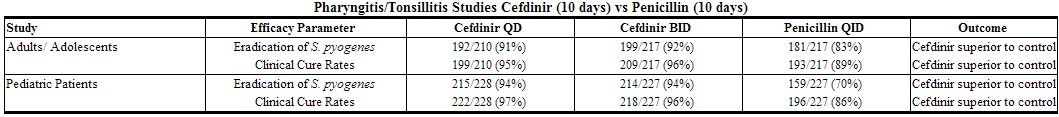 |
Two studies (one in adults and adolescents, the other in pediatric patients) compared 5 days of cefdinir BID to 10 days of penicillin 250 mg or 10 mg/kg QID. Using strict evaluability and microbiologic/ clinical response criteria 4 to 10 days posttherapy, the following clinical cure rates, microbiologic eradication rates, and statistical outcomes were obtained:[1]
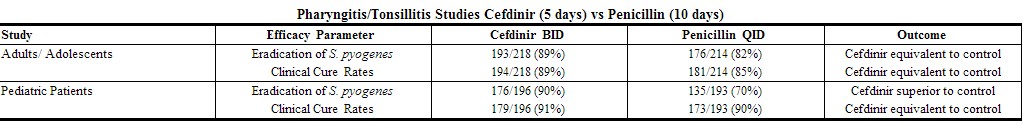 |
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50739S2LBL.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.