Didanosine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: PANCREATITIS, LACTIC ACIDOSIS AND HEPATOMEGALY WITH STEATOSIS
See full prescribing information for complete Boxed Warning.
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk.
|
Overview
Didanosine is a nucleoside reverse transcriptase inhibitor that is FDA approved for the treatment of human immunodeficiency virus (HIV)-1 infection.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, abdominal pain, diarrhea, nausea, vomiting and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Didanosine in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus (HIV)-1 infection.
- Should be administered on an empty stomach
- Total daily dose is based on body weight
- 20 kg to less than 25 kg: 200 mg once daily
- 25 kg to less than 60 kg: 250 mg once daily
- At least 60 kg: 400 mg once daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Didanosine in adult patients.
Non–Guideline-Supported Use
- Prophylaxis of occupational exposure to HIV
- Didanosine: 400 mg (if body weight is less than 60 kg, 125 mg twice daily or 250 mg once daily) daily, on an empty stomach, AND
- Lamivudine: 300 mg once daily or 150 mg twice daily for 4 weeks.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Didanosine in combination with other antiretroviral agents is indicated for the treatment of human immunodeficiency virus (HIV)-1 infection.
- Should be administered on an empty stomach
- Total daily dose is based on body weight
- 20 kg to less than 25 kg: 200 mg once daily
- 25 kg to less than 60 kg: 250 mg once daily
- At least 60 kg: 400 mg once daily
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Didanosine in pediatric patients.
Non–Guideline-Supported Use
- Prophylaxis of occupational exposure to HIV
- Didanosine: 400 mg (if body weight is less than 60 kg, 125 mg twice daily or 250 mg once daily) daily, on an empty stomach, AND
- Lamivudine: 300 mg once daily or 150 mg twice daily for 4 weeks.
Contraindications
These recommendations are based on either drug interaction studies or observed clinical toxicities.
Allopurinol
- Coadministration of didanosine and allopurinol is contraindicated because systemic exposures of didanosine are increased, which may increase didanosine-associated toxicity.
Ribavirin
- Coadministration of didanosine and ribavirin is contraindicated because exposures of the active metabolite of didanosine (dideoxyadenosine 5’-triphosphate) are increased. Fatal hepatic failure, as well as peripheral neuropathy, pancreatitis, and symptomatic hyperlactatemia/lactic acidosis have been reported in patients receiving both didanosine and ribavirin.
Warnings
|
WARNING: PANCREATITIS, LACTIC ACIDOSIS AND HEPATOMEGALY WITH STEATOSIS
See full prescribing information for complete Boxed Warning.
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with suspected pancreatitis and discontinued in patients with confirmed pancreatitis.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk.
|
Pancreatitis
Fatal and nonfatal pancreatitis has occurred during therapy with didanosine used alone or in combination regimens in both treatment-naive and treatment-experienced patients, regardless of degree of immunosuppression. Didanosine delayed-release capsules should be suspended in patients with signs or symptoms of pancreatitis and discontinued in patients with confirmed pancreatitis. Patients treated with didanosine delayed-release capsules in combination with stavudine may be at increased risk for pancreatitis.
When treatment with life-sustaining drugs known to cause pancreatic toxicity is required, suspension of didanosine delayed-release capsules therapy is recommended. In patients with risk factors for pancreatitis, didanosine delayed-release capsules should be used with extreme caution and only if clearly indicated. Patients with advanced HIV-1 infection, especially the elderly, are at increased risk of pancreatitis and should be followed closely. Patients with renal impairment may be at greater risk for pancreatitis if treated without dose adjustment. The frequency of pancreatitis is dose related.
Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals. A majority of these cases have been in women. Obesity and prolonged nucleoside exposure may be risk factors. Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk. Particular caution should be exercised when administering didanosine delayed-release capsules to any patient with known risk factors for liver disease; however, cases have also been reported in patients with no known risk factors. Treatment with didanosine delayed-release capsules should be suspended in any patient who develops clinical signs or symptoms with or without laboratory findings consistent with symptomatic hyperlactatemia, lactic acidosis, or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Hepatic Toxicity
The safety and efficacy of didanosine delayed-release capsules have not been established in HIV-infected patients with significant underlying liver disease. During combination antiretroviral therapy, patients with preexisting liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities, including severe and potentially fatal hepatic adverse events, and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Hepatotoxicity and hepatic failure resulting in death were reported during postmarketing surveillance in HIV-infected patients treated with hydroxyurea and other antiretroviral agents. Fatal hepatic events were reported most often in patients treated with the combination of hydroxyurea, didanosine, and stavudine. This combination should be avoided.
Non-cirrhotic Portal Hypertension
Postmarketing cases of non-cirrhotic portal hypertension have been reported, including cases leading to liver transplantation or death. Cases of didanosine-associated non-cirrhotic portal hypertension were confirmed by liver biopsy in patients with no evidence of viral hepatitis. Onset of signs and symptoms ranged from months to years after start of didanosine therapy. Common presenting features included elevated liver enzymes, esophageal varices, hematemesis, ascites, and splenomegaly.
Patients receiving didanosine delayed-release capsules should be monitored for early signs of portal hypertension (e.g., thrombocytopenia and splenomegaly) during routine medical visits. Appropriate laboratory testing including liver enzymes, serum bilirubin, albumin, complete blood count, and international normalized ratio (INR) and ultrasonography should be considered. Didanosine delayed-release capsules should be discontinued in patients with evidence of non-cirrhotic portal hypertension.
Peripheral Neuropathy
Peripheral neuropathy, manifested by numbness, tingling, or pain in the hands or feet, has been reported in patients receiving didanosine therapy. Peripheral neuropathy has occurred more frequently in patients with advanced HIV disease, in patients with a history of neuropathy, or in patients being treated with neurotoxic drug therapy, including stavudine. Discontinuation of didanosine delayed-release capsules should be considered in patients who develop peripheral neuropathy.
Retinal Changes and Optic Neuritis
Retinal changes and optic neuritis have been reported in patients taking didanosine. Periodic retinal examinations should be considered for patients receiving didanosine delayed-release capsules.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including didanosine delayed-release capsules. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance” have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
Study AI454-152 was a 48-week, randomized, open-label study comparing didanosine delayed-release capsules (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients. Selected clinical adverse reactions that occurred in combination with other antiretroviral agents are provided in TABLE 3.
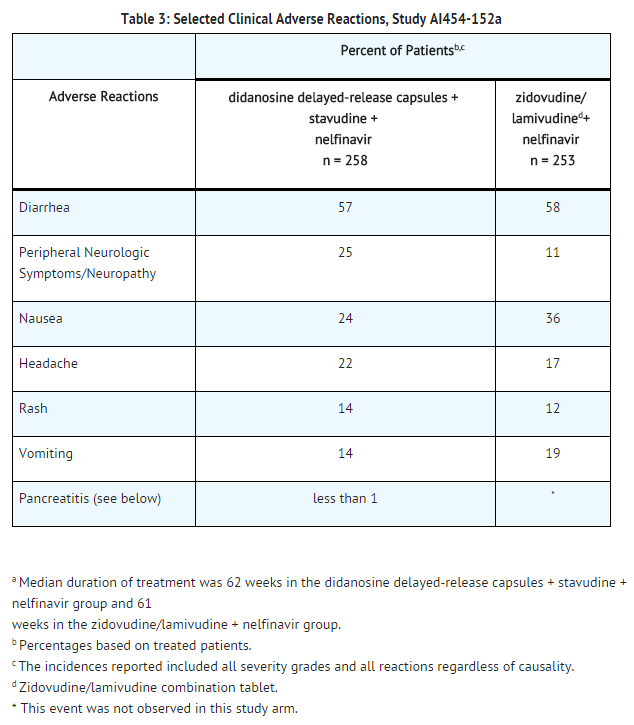
In clinical trials using a buffered formulation of didanosine, pancreatitis resulting in death was observed in one patient who received didanosine plus stavudine plus nelfinavir, one patient who received didanosine plus stavudine plus indinavir, and 2 of 68 patients who received didanosine plus stavudine plus indinavir plus hydroxyurea. In an early access program, pancreatitis resulting in death was observed in one patient who received didanosine delayed-release capsules plus stavudine plus hydroxyurea plus ritonavir plus indinavir plus efavirenz.
The frequency of pancreatitis is dose related. In phase 3 studies with buffered formulations of didanosine, incidence ranged from 1% to 10% with doses higher than are currently recommended and 1% to 7% with recommended dose.
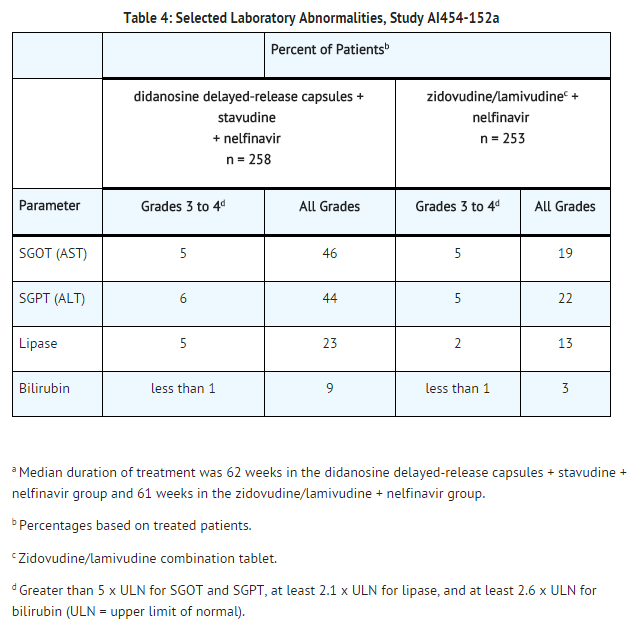
Selected laboratory abnormalities that occurred in a study of didanosine delayed-release capsules in combination with other antiretroviral agents are shown in TABLE 4.
Pediatric Patients
In clinical trials, 743 pediatric patients between 2 weeks and 18 years of age have been treated with didanosine. Adverse reactions and laboratory abnormalities reported to occur in these patients were generally consistent with the safety profile of didanosine in adults.
In pediatric phase 1 studies, pancreatitis occurred in 2 of 60 (3%) patients treated at entry doses below 300 mg/m2/day and in 5 of 38 (13%) patients treated at higher doses. In study ACTG 152, pancreatitis occurred in none of the 281 pediatric patients who received didanosine 120 mg/m2 every 12 hours and in less than 1% of the 274 pediatric patients who received didanosine 90 mg/m2 every 12 hours in combination with zidovudine.
Retinal changes and optic neuritis have been reported in pediatric patients.
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of didanosine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to didanosine, or a combination of these factors.
- Blood and Lymphatic System Disorders - anemia, leukopenia, and thrombocytopenia.
- Body as a Whole - abdominal pain, alopecia, anaphylactoid reaction, asthenia, chills/fever, pain, and redistribution/accumulation of body fat.
- Digestive Disorders - anorexia, dyspepsia, and flatulence.
- Exocrine Gland Disorders - pancreatitis (including fatal cases), sialoadenitis, parotid gland enlargement, dry mouth, and dry eyes.
- Hepatobiliary Disorders - symptomatic hyperlactatemia/lactic acidosis and hepatic steatosis; non-cirrhotic portal hypertension; hepatitis and liver failure.
- Metabolic Disorders - diabetes mellitus, elevated serum alkaline phosphatase level, elevated serum amylase level, elevated serum gamma-glutamyltransferase level, elevated serum uric acid level, hypoglycemia, and hyperglycemia.
- Musculoskeletal Disorders - myalgia (with or without increases in creatine kinase), rhabdomyolysis including acute renal failure and hemodialysis, arthralgia, and myopathy.
- Ophthalmologic Disorders - retinal depigmentation and optic neuritis.
Use with Stavudine- and Hydroxyurea-Based Regimens
When didanosine is used in combination with other agents with similar toxicities, the incidence of these toxicities may be higher than when didanosine is used alone. Thus, patients treated with didanosine delayed-release capsules in combination with stavudine, with or without hydroxyurea, may be at increased risk for pancreatitis and hepatotoxicity, which may be fatal, and severe peripheral neuropathy. The combination of didanosine delayed-release capsules and hydroxyurea, with or without stavudine, should be avoided.
Drug Interactions
Established Drug Interactions
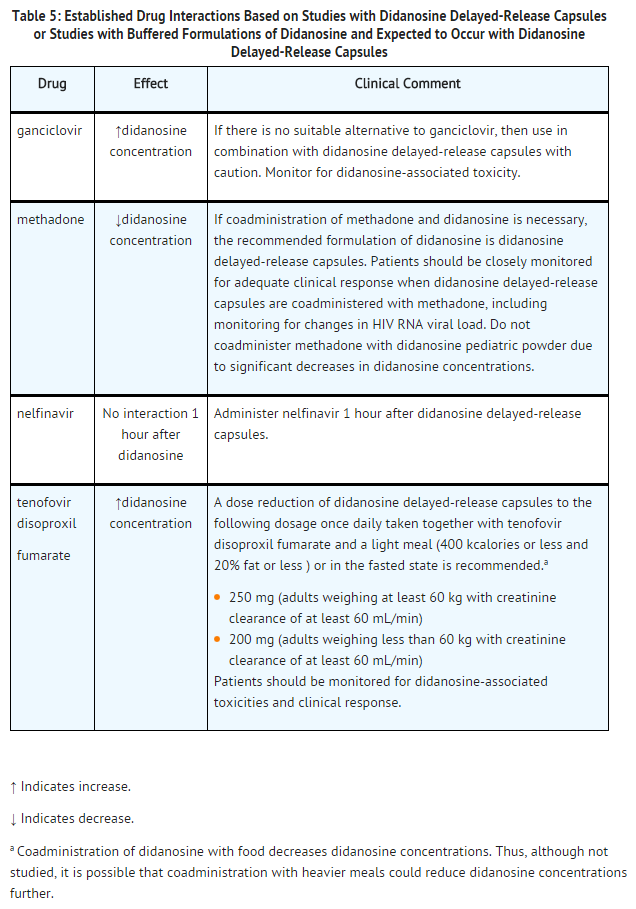
Exposure to didanosine is increased when coadministered with tenofovir disoproxil fumarate. Increased exposure may cause or worsen didanosine-related clinical toxicities, including pancreatitis, symptomatic hyperlactatemia/lactic acidosis, and peripheral neuropathy. Coadministration of tenofovir disoproxil fumarate with didanosine delayed-release capsules should be undertaken with caution, and patients should be monitored closely for didanosine-related toxicities and clinical response. Didanosine delayed-release capsules should be suspended if signs or symptoms of pancreatitis, symptomatic hyperlactatemia, or lactic acidosis develop. Suppression of CD4 cell counts has been observed in patients receiving tenofovir disoproxil fumarate with didanosine at a dose of 400 mg daily.
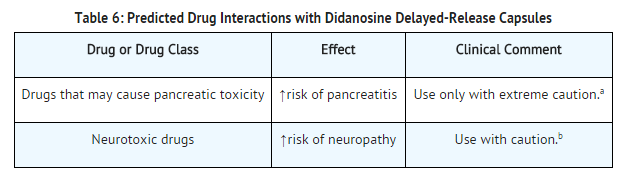
Predicted Drug Interactions
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Reproduction studies have been performed in rats and rabbits at doses up to 12 and 14.2 times the estimated human exposure (based upon plasma levels), respectively, and have revealed no evidence of impaired fertility or harm to the fetus due to didanosine. At approximately 12 times the estimated human exposure, didanosine was slightly toxic to female rats and their pups during mid and late lactation. These rats showed reduced food intake and body weight gains but the physical and functional development of the offspring was not impaired and there were no major changes in the F2 generation. A study in rats showed that didanosine and/or its metabolites are transferred to the fetus through the placenta. Animal reproduction studies are not always predictive of human response.
There are no adequate and well-controlled studies of didanosine in pregnant women. Didanosine should be used during pregnancy only if the potential benefit justifies the potential risk.
Fatal lactic acidosis has been reported in pregnant women who received the combination of didanosine and stavudine with other antiretroviral agents. It is unclear if pregnancy augments the risk of lactic acidosis/hepatic steatosis syndrome reported in nonpregnant individuals receiving nucleoside analogues. The combination of didanosine and stavudine should be used with caution during pregnancy and is recommended only if the potential benefit clearly outweighs the potential risk. Healthcare providers caring for HIV-infected pregnant women receiving didanosine should be alert for early diagnosis of lactic acidosis/hepatic steatosis syndrome.
Pregnancy Category (AUS): B2
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Didanosine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Didanosine during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. A study in rats showed that following oral administration, didanosine and/or its metabolites were excreted into the milk of lactating rats. It is not known if didanosine is excreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving didanosine.
Pediatric Use
Use of didanosine in pediatric patients from 2 weeks of age through adolescence is supported by evidence from adequate and well-controlled studies of didanosine in adult and pediatric patients. Additional pharmacokinetic studies in pediatric patients support use of didanosine delayed-release capsules in pediatric patients who weigh at least 20 kg.
Geriatic Use
In an Expanded Access Program using a buffered formulation of didanosine for the treatment of advanced HIV infection, patients aged 65 years and older had a higher frequency of pancreatitis (10%) than younger patients (5%). Clinical studies of didanosine, including those for didanosine delayed-release capsules, did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently than younger subjects. Didanosine is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection. In addition, renal function should be monitored and dosage adjustments should be made accordingly.
Gender
The effects of gender on didanosine pharmacokinetics have not been studied.
Race
There is no FDA guidance on the use of Didanosine with respect to specific racial populations.
Renal Impairment
Patients with renal impairment (creatinine clearance of less than 60 mL/min) may be at greater risk of toxicity from didanosine due to decreased drug clearance. A dose reduction is recommended for these patients.
Hepatic Impairment
The pharmacokinetics of didanosine have been studied in 12 non-HIV-infected subjects with moderate (n = 8) to severe (n = 4) hepatic impairment (Child-Pugh Class B or C). Mean AUC and Cmax values following a single 400 mg dose of didanosine were approximately 13% and 19% higher, respectively, in patients with hepatic impairment compared to matched healthy subjects. No dose adjustment is needed, because a similar range and distribution of AUC and Cmax values was observed for subjects with hepatic impairment and matched healthy controls.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Didanosine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Didanosine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Didanosine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Didanosine and IV administrations.
Overdosage
There is no known antidote for didanosine overdosage. In phase 1 studies, in which buffered formulations of didanosine were initially administered at doses ten times the currently recommended dose, toxicities included: pancreatitis, peripheral neuropathy, diarrhea, hyperuricemia, and hepatic dysfunction. Didanosine is not dialyzable by peritoneal dialysis, although there is some clearance by hemodialysis.
Pharmacology
| |
Didanosine
| |
| Systematic (IUPAC) name | |
| 9-((2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl)-3H-purin-6(9H)-one | |
| Identifiers | |
| CAS number | |
| ATC code | J05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 236.227 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 30 to 54% |
| Protein binding | Less than 5% |
| Metabolism | ? |
| Half life | 1.5 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
Didanosine is a synthetic nucleoside analogue of the naturally occurring nucleoside deoxyadenosine in which the 3’-hydroxyl group is replaced by hydrogen. Intracellularly, didanosine is converted by cellular enzymes to the active metabolite, dideoxyadenosine 5’-triphosphate. Dideoxyadenosine 5’-triphosphate inhibits the activity of HIV-1 reverse transcriptase both by competing with the natural substrate, deoxyadenosine 5’-triphosphate, and by its incorporation into viral DNA causing termination of viral DNA chain elongation.
Structure
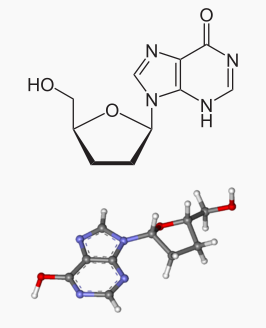
The chemical name for didanosine is 2' ,3' -dideoxyinosine. The structural formula is:
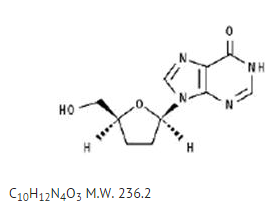
Pharmacodynamics
There is limited information regarding Didanosine Pharmacodynamics in the drug label.
Pharmacokinetics
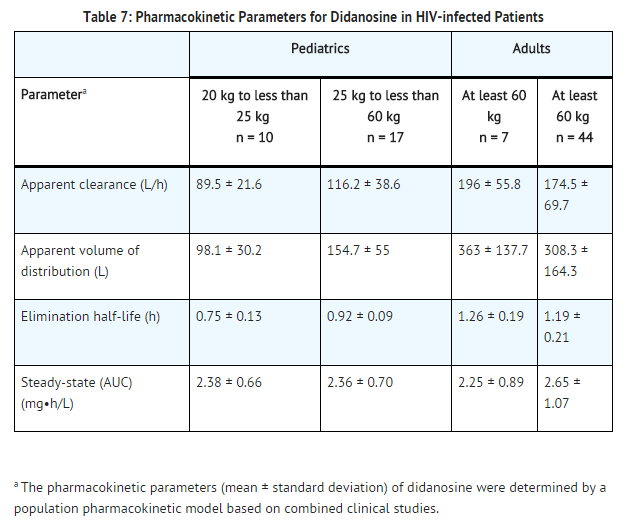
The pharmacokinetic parameters of didanosine in HIV-infected adult and pediatric patients are summarized in TABLE 7, by weight ranges that correspond to recommended doses. Didanosine is rapidly absorbed, with peak plasma concentrations generally observed from 0.25 to 1.50 hours following oral dosing with a buffered formulation. Increases in plasma didanosine concentrations were dose proportional over the range of 50 to 400 mg. In adults, the mean (± standard deviation) oral bioavailability following single oral dosing with a buffered formulation is 42 (± 12)%. After oral administration, the urinary recovery of didanosine is approximately 18 (± 8)% of the dose. The CSF-plasma ratio following IV administration is 21 (± 0.03)%. Steady-state pharmacokinetic parameters did not differ significantly from values obtained after a single dose. Binding of didanosine to plasma proteins in vitro was low (less than 5%). Based on data from in vitro and animal studies, it is presumed that the metabolism of didanosine in man occurs by the same pathways responsible for the elimination of endogenous purines.
Comparison of Didanosine Formulations
In didanosine delayed-release capsules, the active ingredient, didanosine, is protected against degradation by stomach acid by the use of an enteric coating on the pellets in the capsule. The enteric coating dissolves when the pellets empty into the small intestine, the site of drug absorption. With buffered formulations of didanosine, administration with antacid provides protection from degradation by stomach acid.
In healthy volunteers, as well as subjects infected with HIV-1, the AUC is equivalent for didanosine administered as the didanosine delayed-release capsules formulation relative to a buffered tablet formulation. The peak plasma concentration (Cmax) of didanosine, administered as didanosine delayed-release capsules, is reduced approximately 40% relative to didanosine buffered tablets. The time to the peak concentration (Tmax) increases from approximately 0.67 hours for didanosine buffered tablets to 2 hours for didanosine delayed-release capsules.
Effect of Food
In the presence of food, the Cmax and AUC for didanosine delayed-release capsules were reduced by approximately 46% and 19%, respectively, compared to the fasting state. Didanosine delayed-release capsules should be taken on an empty stomach.
Nonclinical Toxicology
Antiviral Activity in Cell Culture
The anti-HIV-1 activity of didanosine was evaluated in a variety of HIV-1 infected lymphoblastic cell lines and monocyte/macrophage cell cultures. The concentration of drug necessary to inhibit viral replication by 50% (EC50) ranged from 2.5 to 10 μM (1 μM = 0.24 mcg/mL) in lymphoblastic cell lines and 0.01 to 0.1 μM in monocyte/macrophage cell cultures.
Resistance
HIV-1 isolates with reduced sensitivity to didanosine have been selected in cell culture and were also obtained from patients treated with didanosine. Genetic analysis of isolates from didanosine-treated patients showed mutations in the reverse transcriptase gene that resulted in the amino acid substitutions K65R, L74V, and M184V. The L74V substitution was most frequently observed in clinical isolates. Phenotypic analysis of HIV-1 isolates from 60 patients (some with prior zidovudine treatment) receiving 6 to 24 months of didanosine monotherapy showed that isolates from 10 of 60 patients exhibited an average of a 10-fold decrease in susceptibility to didanosine in cell culture compared to baseline isolates. Clinical isolates that exhibited a decrease in didanosine susceptibility harbored one or more didanosine resistance-associated substitutions.
Cross-resistance
HIV-1 isolates from 2 of 39 patients receiving combination therapy for up to 2 years with didanosine and zidovudine exhibited decreased susceptibility to didanosine, lamivudine, stavudine, zalcitabine, and zidovudine in cell culture. These isolates harbored five substitutions (A62V, V75I, F77L, F116Y, and Q151M) in the reverse transcriptase gene. In data from clinical studies, the presence of thymidine analogue mutations (M41L, D67N, L210W, T215Y, K219Q) has been shown to decrease the response to didanosine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were conducted in mice and rats for 22 and 24 months, respectively. In the mouse study, initial doses of 120, 800, and 1200 mg/kg/day for each sex were lowered after 8 months to 120, 210, and 210 mg/kg/day for females and 120, 300, and 600 mg/kg/day for males. The two higher doses exceeded the maximally tolerated dose in females and the high dose exceeded the maximally tolerated dose in males. The low dose in females represented 0.68-fold maximum human exposure and the intermediate dose in males represented 1.7-fold maximum human exposure based on relative AUC comparisons. In the rat study, initial doses were 100, 250, and 1000 mg/kg/day, and the high dose was lowered to 500 mg/kg/day after 18 months. The upper dose in male and female rats represented 3-fold maximum human exposure.
Didanosine induced no significant increase in neoplastic lesions in mice or rats at maximally tolerated doses.
Didanosine was positive in the following genetic toxicology assays: 1) the Escherichia coli tester strain WP2 uvrA bacterial mutagenicity assay; 2) the L5178Y/TK+/- mouse lymphoma mammalian cell gene mutation assay; 3) the in vitro chromosomal aberrations assay in cultured human peripheral lymphocytes; 4) the in vitro chromosomal aberrations assay in Chinese Hamster Lung cells; and 5) the BALB/c 3T3 in vitro transformation assay. No evidence of mutagenicity was observed in an Ames Salmonella bacterial mutagenicity assay or in rat and mouse in vivo micronucleus assays.
Animal Toxicology and/or Pharmacology
Evidence of a dose-limiting skeletal muscle toxicity has been observed in mice and rats (but not in dogs) following long-term (greater than 90 days) dosing with didanosine at doses that were approximately 1.2 to 12 times the estimated human exposure. The relationship of this finding to the potential of didanosine to cause myopathy in humans is unclear. However, human myopathy has been associated with administration of didanosine and other nucleoside analogues.
Clinical Studies
Adult Patients
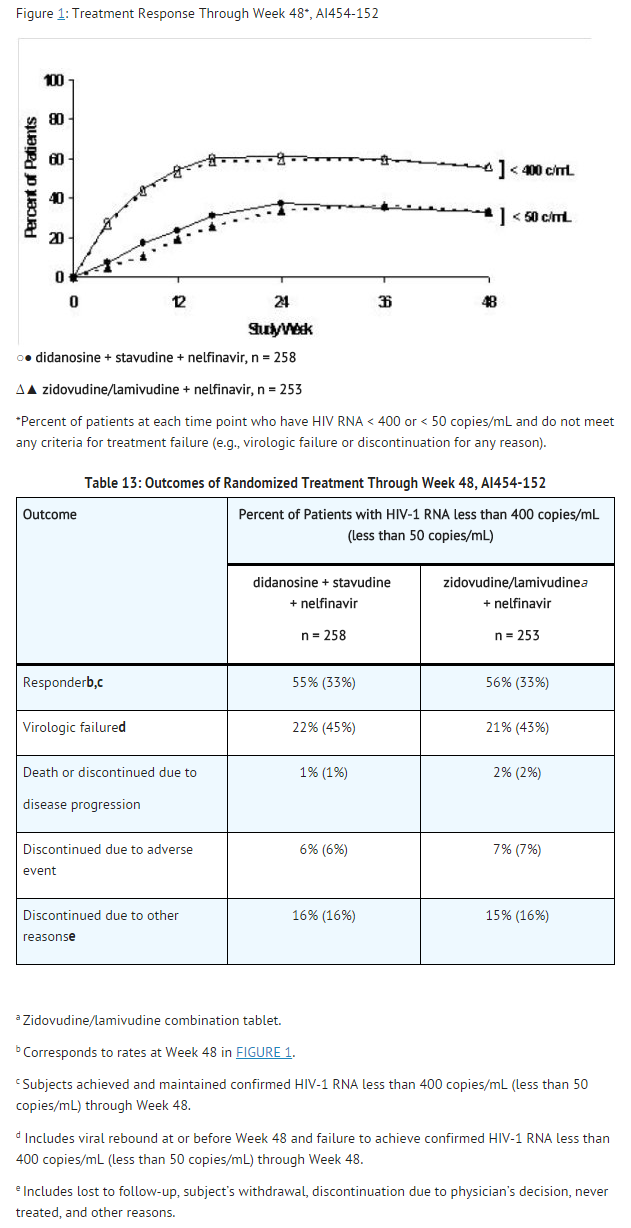
Study AI454-152 was a 48-week, randomized, open-label study comparing didanosine delayed-release capsules (400 mg once daily) plus stavudine (40 mg twice daily) plus nelfinavir (750 mg three times daily) to zidovudine (300 mg) plus lamivudine (150 mg) combination tablets twice daily plus nelfinavir (750 mg three times daily) in 511 treatment-naive patients, with a mean CD4 cell count of 411 cells/mm3 (range 39 to 1105 cells/mm3) and a mean plasma HIV-1 RNA of 4.71 log10 copies/mL (range 2.8 to 5.9 log10 copies/mL) at baseline. Patients were primarily males (72%) and Caucasian (53%) with a mean age of 35 years (range 18 to 73 years). The percentages of patients with HIV-1 RNA less than 400 and less than 50 copies/mL and outcomes of patients through 48 weeks are summarized in FIGURE 1 and TABLE 13, respectively.
Pediatric Patients
Efficacy in pediatric patients was demonstrated in a randomized, double-blind, controlled study (ACTG 152, conducted 1991-1995) involving 831 patients 3 months to 18 years of age treated for more than 1.5 years with zidovudine (180 mg/m2 every 6 hours), didanosine (120 mg/m2 every 12 hours), or zidovudine (120 mg/m2 every 6 hours) plus didanosine (90 mg/m2 every 12 hours). Patients treated with didanosine or didanosine plus zidovudine had lower rates of HIV-1 disease progression or death compared with those treated with zidovudine alone.
How Supplied
- Didanosine 200 mg: Bottles of 30 capsules.
- Didanosine 250 mg: Bottles of 30 capsules.
- Didanosine 400 mg: Bottles of 30 capsules.
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Didanosine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
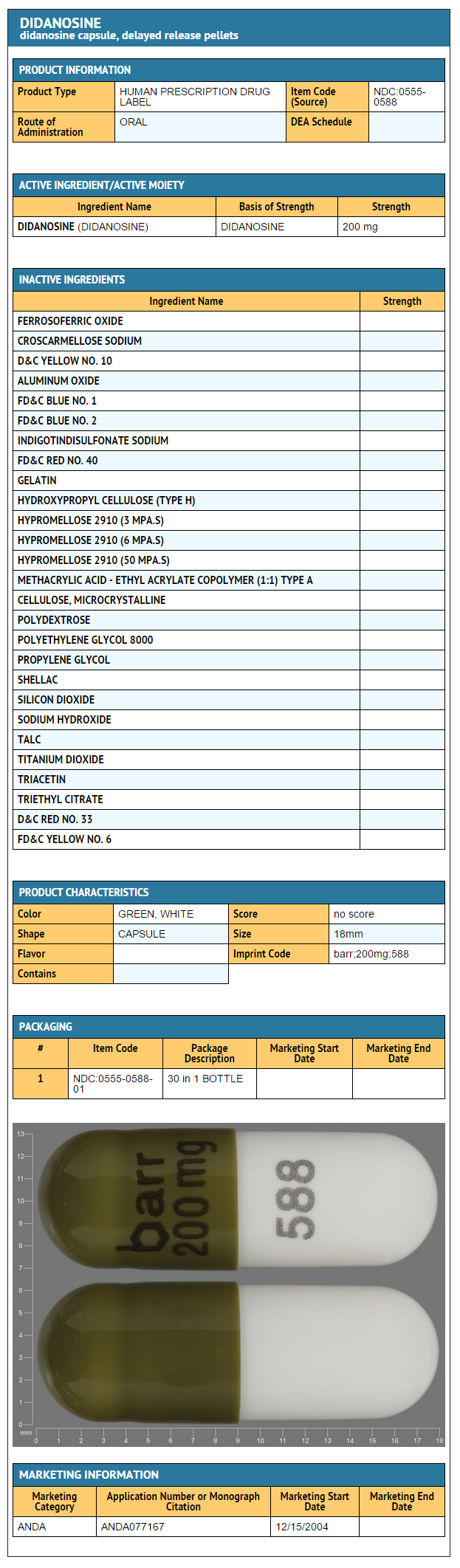
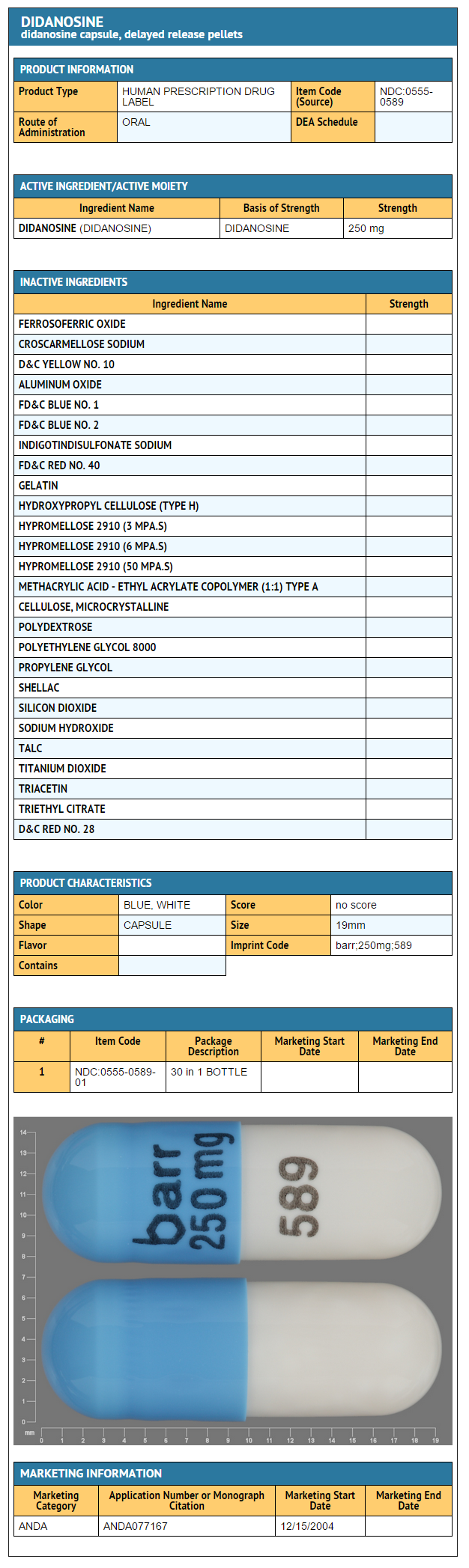
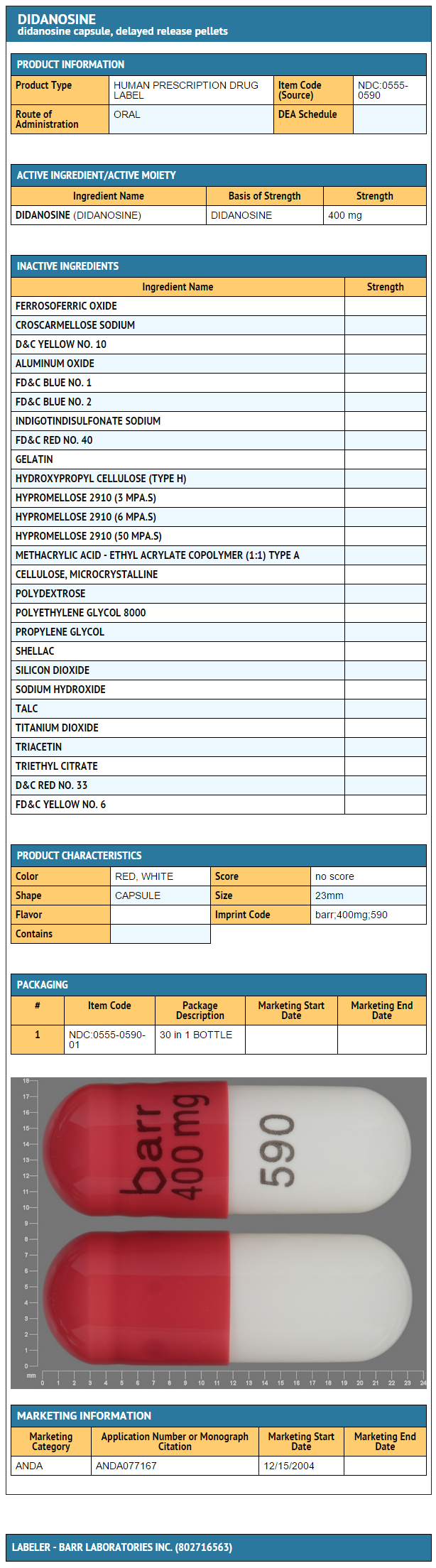
{{#ask: Label Page::Didanosine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Pancreatitis
Patients should be informed that a serious toxicity of didanosine, used alone and in combination regimens, is pancreatitis, which may be fatal.
Peripheral Neuropathy
Patients should be informed that peripheral neuropathy, manifested by numbness, tingling, or pain in hands or feet, may develop during therapy with didanosine delayed-release capsules. Patients should be counseled that peripheral neuropathy occurs with greatest frequency in patients with advanced HIV-1 disease or a history of peripheral neuropathy, and discontinuation of didanosine delayed-release capsules may be required if toxicity develops.
Lactic Acidosis and Severe Hepatomegaly with Steatosis
Patients should be informed that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogues alone or in combination, including didanosine and other antiretrovirals.
Hepatic Toxicity
Patients should be informed that hepatotoxicity including fatal hepatic adverse events were reported in patients with preexisting liver dysfunction. The safety and efficacy of didanosine delayed-release capsules has not been established in HIV-infected patients with significant underlying liver disease.
Non-cirrhotic Portal Hypertension
Patients should be informed that non-cirrhotic portal hypertension has been reported in patients taking didanosine delayed-release capsules, including cases leading to liver transplantation or death.
Retinal Changes and Optic Neuritis
Patients should be informed that retinal changes and optic neuritis have been reported in adult and pediatric patients.
Fat Redistribution
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
Concomitant Therapy
Patients should be informed that when didanosine is used in combination with other agents with similar toxicities, the incidence of adverse events may be higher than when didanosine is used alone. These patients should be followed closely.
Patients should be cautioned about the use of medications or other substances, including alcohol, which may exacerbate didanosine delayed-release capsules toxicities.
General Information
Didanosine delayed-release capsules are not a cure for HIV-1 infection, and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Therefore, patients should remain under the care of a physician when using didanosine delayed-release capsules.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom or other barrier method to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. It is not known if didanosine can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breast-feed because HIV-1 can be passed to the baby in breast milk.
Patients should be instructed to swallow the capsule as a whole and to not open the capsule.
Patients should be instructed to not miss a dose but if they do, patients should take didanosine as soon as possible. Patients should be told that if it is almost time for the next dose, they should skip the missed dose and continue with the regular dosing schedule.
Patients should be instructed to contact a poison control center or emergency room right away in case of an overdose.
Precautions with Alcohol
Alcohol-Didanosine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Videx [1]
- Videx EC
- Videx Pediatric
Look-Alike Drug Names
There is limited information regarding Didanosine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Didanosine |Label Name=Didanosine 200 mg.png
}}
{{#subobject:
|Label Page=Didanosine |Label Name=Didanosine 250 mg.png
}}
{{#subobject:
|Label Page=Didanosine |Label Name=Didanosine 400 mg.png
}}