Ultraviolet
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than soft X-rays. It is so named because the spectrum consists of electromagnetic waves with frequencies higher than those that humans identify as the color violet (purple).
UV light is typically found as part of the radiation received by the Earth from the Sun. Most humans are aware of the effects of UV through the painful condition of sunburn. The UV spectrum has many other effects, including both beneficial and damaging changes to human health.
Discovery
The discovery of UV radiation was intimately associated with the observation that silver salts darken when exposed to sunlight. In 1801 the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum were especially effective at darkening silver chloride-soaked paper. He called them "de-oxidizing rays" to emphasize their chemical reactivity and to distinguish them from "heat rays" at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favor of ultraviolet and infrared radiation, respectively.[1]
Origin of term
The name means "beyond violet" (from Latin ultra, "beyond"), violet being the color of the shortest wavelengths of visible light. UV light has a shorter wavelength than that of violet light.
Subtypes
The electromagnetic spectrum of ultraviolet light can be subdivided in a number of ways. The draft ISO standard on determining solar irradiances (ISO-DIS-21348)[2] describes the following ranges:
| Name | Abbreviation | Wavelength range in nanometers | Energy per photon |
|---|---|---|---|
| Ultraviolet A, long wave, or black light | UVA | 400 nm - 315 nm | 3.10 - 3.94 eV |
| Near | NUV | 400 nm - 300 nm | 3.10 - 4.13 eV |
| Ultraviolet B or medium wave | UVB | 315 nm - 280 nm | 3.94 - 4.43 eV |
| Middle | MUV | 300 nm - 200 nm | 4.13 - 6.20 eV |
| Ultraviolet C, short wave, or germicidal | UVC | 280 nm - 100 nm | 4.43 - 12.4 eV |
| Far | FUV | 200 nm - 122 nm | 6.20 - 10.2 eV |
| Vacuum | VUV | 200 nm - 10 nm | 6.20 - 124 eV |
| Extreme | EUV | 121 nm - 10 nm | 10.2 - 124 eV |
In photolithography, in laser technology, etc., the term deep ultraviolet or DUV refers to wavelengths below 300 nm. "Vacuum UV" is so named because it is absorbed strongly by air and is therefore used in a vacuum. In the long-wave limit of this region, roughly 150-200 nm, the principal absorber is the oxygen in air. Work in this region can be performed in an oxygen free atmosphere, pure nitrogen being commonly used, which avoids the need for a vacuum chamber.
See 1 E-7 m for a list of objects of comparable sizes.
Black light
A black light, or Wood's light, is a lamp that emits long wave UV radiation and very little visible light. Commonly these are referred to as simply a "UV light". Fluorescent black lights are typically made in the same fashion as normal fluorescent lights except that only one phosphor is used and the normally clear glass envelope of the bulb may be replaced by a deep-bluish-purple glass called Wood's glass, a nickel-oxide–doped glass, which blocks almost all visible light above 400 nanometers. The color of such lamps is often referred to in the trade as "blacklight blue" or "BLB." This is to distinguish these lamps from "bug zapper" blacklight ("BL") lamps that don't have the blue Wood's glass. The phosphor typically used for a near 368 to 371 nanometer emission peak is either europium-doped strontium fluoroborate (SrB4O7F:Eu2+) or europium-doped strontium borate (SrB4O7:Eu2+) while the phosphor used to produce a peak around 350 to 353 nanometers is lead-doped barium silicate (BaSi2O5:Pb+). "Blacklight Blue" lamps peak at 365 nm.
While "black lights" do produce light in the UV range, their spectrum is confined to the longwave UVA region. Unlike UVB and UVC, which are responsible for the direct DNA damage that leads to skin cancer, black light is limited to lower energy, longer waves and does not cause sunburn. However, UVA is capable of causing damage to collagen fibers and destroying vitamin A in skin.
A black light may also be formed by simply using Wood's glass instead of clear glass as the envelope for a common incandescent bulb. This was the method used to create the very first black light sources. Though it remains a cheaper alternative to the fluorescent method, it is exceptionally inefficient at producing UV light (a mere few lumens per watt) owing to the black body nature of the incandescent light source. Incandescent UV bulbs, due to their inefficiency, may also become dangerously hot during use. More rarely still, high power (hundreds of watts) mercury vapor black lights can be found which use a UV emitting phosphor and an envelope of Wood's glass. These lamps are used mainly for theatrical and concert displays and also become very hot during normal use.
Some UV fluorescent bulbs specifically designed to attract insects for use in bug zappers use the same near-UV emitting phosphor as normal blacklights, but use plain glass instead of the more expensive Wood's glass. Plain glass blocks less of the visible mercury emission spectrum, making them appear light blue to the naked eye. These lamps are referred to as "blacklight" or "BL" in most lighting catalogs.
Ultraviolet light can be also generated by some light-emitting diodes.
Natural sources of UV
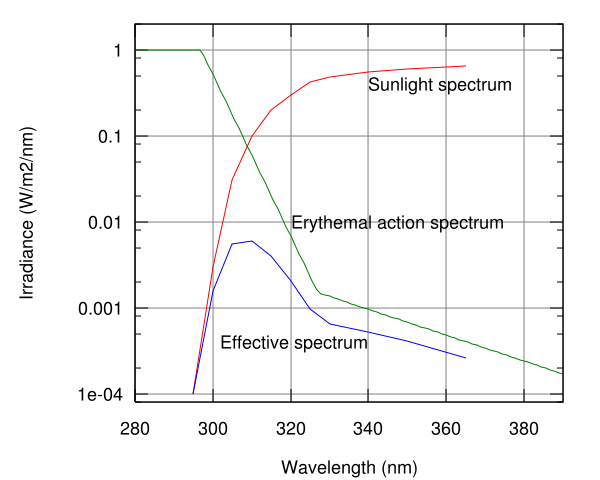
The Sun emits ultraviolet radiation in the UVA, UVB, and UVC bands, but because of absorption in the atmosphere's ozone layer, 98.7% of the ultraviolet radiation that reaches the Earth's surface is UVA. (Some of the UVB and UVC radiation is responsible for the generation of the ozone layer.)
Ordinary glass is partially transparent to UVA but is opaque to shorter wavelengths while Silica or quartz glass, depending on quality, can be transparent even to vacuum UV wavelengths. Ordinary window glass passes about 90% of the light above 350 nm, but blocks over 90% of the light below 300 nm.[3][4][5]
The onset of vacuum UV, 200 nm, is defined by the fact that ordinary air is opaque below this wavelength. This opacity is due to the strong absorption of light of these wavelengths by oxygen in the air. Pure nitrogen (less than about 10 ppm oxygen) is transparent to wavelengths in the range of about 150–200 nm. This has wide practical significance now that semiconductor manufacturing processes are using wavelengths shorter than 200 nm. By working in oxygen-free gas, the equipment does not have to be built to withstand the pressure differences required to work in a vacuum. Some other scientific instruments, such as circular dichroism spectrometers, are also commonly nitrogen purged and operate in this spectral region.
Extreme UV is characterized by a transition in the physics of interaction with matter: wavelengths longer than about 30 nm interact mainly with the chemical valence electrons of matter, while wavelengths shorter than that interact mainly with inner shell electrons and nuclei. The long end of the EUV/XUV spectrum is set by a prominent He+ spectral line at 30.4nm. XUV is strongly absorbed by most known materials, but it is possible to synthesize multilayer optics that reflect up to about 50% of XUV radiation at normal incidence. This technology has been used to make telescopes for solar imaging; it was pioneered by the NIXT and MSSTA sounding rockets in the 1990s; (current examples are SOHO/EIT and TRACE) and for nanolithography (printing of traces and devices on microchips).
Beneficial effects
The Earth's atmosphere blocks UV radiation from penetrating through the atmosphere by 98.7%. A positive effect of UVB exposure is that it induces the production of vitamin D in the skin. It has been estimated that tens of thousands of premature deaths occur in the United States annually from a range of cancers due to vitamin D deficiency.[6] Another effect of vitamin D deficiency is osteomalacia (the adult equivalent of rickets), which can result in bone pain, difficulty in weight bearing and sometimes fractures. Other studies show most people get adequate Vitamin D through food and incidental exposure.[7]
Many countries have fortified certain foods with Vitamin D to prevent deficiency. Eating fortified foods or taking a dietary supplement pill is usually preferred to UVB exposure, due to the increased risk of skin cancer from UV radiation.[7]
Too little UVB radiation leads to a lack of Vitamin D. Too much UVB radiation leads to direct DNA damages and sunburn. An appropriate amount of UVB (What is appropriate depends on your skin colour) leads to a limited amount of direct DNA damage. This is recognized and repaired by the body. Then the melanin production is increased which leads to a long lasting tan. This tan occurs with a 2 day lag phase after irradiation, but it is much less harmful and long lasting than the one obtained from UVA.
Ultraviolet radiation has other medical applications, in the treatment of skin conditions such as psoriasis and vitiligo. UVA radiation can be used in conjunction with psoralens (PUVA treatment). UVB radiation is rarely used in conjunction with psoralens. In cases of psoriasis and vitiligo, UV light with wavelength of 311 nm is most effective.
Harmful effects
An overexposure to UVB radiation can cause sunburn and some forms of skin cancer. In humans, prolonged exposure to solar UV radiation may result in acute and chronic health effects on the skin, eye, and immune system.[8] However the most deadly form - malignant melanoma - is mostly caused by the indirect DNA damage (free radicals and oxidative stress). This can be seen from the absence of a UV-signature mutation in 92% of all melanoma.[9]
UVC rays are the highest energy, most dangerous type of ultraviolet light. Little attention has been given to UVC rays in the past since they are filtered out by the atmosphere. However, their use in equipment such as pond sterilization units may pose an exposure risk, if the lamp is switched on outside of its enclosed pond sterilization unit.
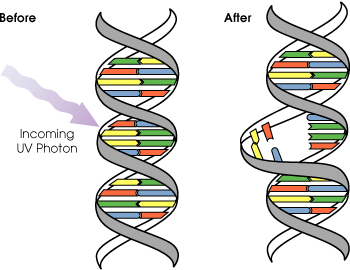
Skin
Template:Cquote2 UVA, UVB and UVC can all damage collagen fibers and thereby accelerate aging of the skin. Both UVA and UVB destroy vitamin A in skin which may cause further damage.[10] In the past UVA was considered less harmful, but today it is known, that it can contribute to skin cancer via the indirect DNA damage (free radicals and reactive oxygen species). It penetrates deeply but it does not cause sunburn. UVA does not damage DNA directly like UVB and UVC, but it can generate highly reactive chemical intermediates, such as hydroxyl and oxygen radicals, which in turn can damage DNA. Because it does not cause reddening of the skin (erythema) it cannot be measured in the SPF testing. There is no good clinical measurement of the blocking of UVA radiation, but it is important that sunscreen block both UVA and UVB. Some scientists blame the absence of UVA filters in sunscreens for the higher melanoma-risk that was found for sunscreen users. [11]
UVB light can cause direct DNA damage. The radiation excites DNA molecules in skin cells, causing covalent bonds to form between adjacent thymine bases, producing thymidine dimers. Thymidine dimers do not base pair normally, which can cause distortion of the DNA helix, stalled replication, gaps, and misincorporation. These can lead to mutations, which can result in cancerous growths. The mutations that are caused by the direct DNA damage carry a UV signature mutation. The mutagenicity of UV radiation can be easily observed in bacteria cultures. This cancer connection is one reason for concern about ozone depletion and the ozone hole. UVB causes some damage to collagen but at a very much slower rate than UVA.
As a defense against UV radiation, the body tans when exposed to moderate (depending on skin type) levels of radiation. UVA gives a quick tan that last for days by oxidizing melanin that was already present and it triggers the release of the melanin from melanocytes. UVB yields a tan that takes roughly 2 days to develop because it stimulates the body to produce more melanin. The purpose of melanin is to block UV-radiation and prevent damage to the vulnerable skin tissues deeper down. The photochemical properties of melanin make it an excellent photoprotectant
Sunscreen prevents the direct DNA damage which causes sunburn. Most of these products contain an SPF rating to show how well they block UVB rays. The SPF rating, however, offers no data about UVA protection. In the US, the FDA is considering adding a star rating system to show UVA protection. A similar system is already used in some European countries.
Some sunscreen lotions now include compounds such as titanium dioxide which helps protect against UVA rays. Other UVA blocking compounds found in sunscreen include zinc oxide and avobenzone. Cantaloupe extract, rich in the compound superoxide dismutase (SOD), can be bound with gliadin to form glisodin, an orally-effective protectant against UVB radiation. There are also naturally occurring compounds found in rainforest plants that have been known to protect the skin from UV radiation damage, such as the fern Phlebodium aureum.
Sunscreen safety debate
The majority of doctors recommend patients protect themselves from UV radiation using sunscreen. Some scientists, however, question the safety of sunscreens that are absorbed into the skin or the bloodstream. These individuals claim these sunscreens generate free radicals under UV-illumination. [12] (See the sunscreen article for a full discussion of this issue.)
Eye
High intensities of UVB light are hazardous to the eyes, and exposure can cause welder's flash (photokeratitis or arc eye) and may lead to cataracts, pterygium,[13][14] and pinguecula formation.
Protective eyewear is beneficial to those who are working with or those who might be exposed to ultraviolet radiation, particularly short wave UV. Given that light may reach the eye from the sides, full coverage eye protection is usually warranted if there is an increased risk of exposure, as in high altitude mountaineering. Mountaineers are exposed to higher than ordinary levels of UV radiation, both because there is less atmospheric filtering and because of reflection from snow and ice.
Ordinary, untreated eyeglasses give some protection. Most plastic lenses give more protection than glass lenses, because, as noted above, glass is transparent to UVA and the common acrylic plastic used for lenses is less so. Some plastic lens materials, such as polycarbonate, inherently block most UV. There are protective treatments available for eyeglass lenses that need it which will give better protection. But even a treatment that completely blocks UV will not protect the eye from light that arrives around the lens.
Degradation of polymers, pigments and dyes
Many polymers used in consumer products are degraded by UV light, and need addition of UV stabilizers to inhibit attack. Products include thermoplastics, such as polypropylene and polyethylene as well as speciality fibres like aramids. UV absorption leads to chain degradation and loss of strength. In addition, many pigments and dyes absorb UV and change colour, so paintings and textiles may need extra protection both from sunlight and fluorescent lamps.
Blockers and absorbers
Ultraviolet Light Absorbers (UVAs) are molecules used in organic materials (polymers, paints, etc.) to absorb UV light in order to reduce the degradation (photo-oxidation) of a material. A number of different UVAs exist with different absorption properties. UVAs can disappear over time, so monitoring of UVA levels in weathered materials is necessary.
In sunscreen, ingredients which absorb UVA/UVB rays, such as avobenzone and octyl methoxycinnamate, are known as absorbers. They are contrasted with physical "blockers" of UV radiation such as titanium dioxide and zinc oxide. (See sunscreen for a more complete list.)
Applications of UV
Spectrophotometry
UV/VIS spectroscopy is widely used as a technique in chemistry, to analyze chemical structure, most notably conjugated systems. UV radiation is often used in visible spectrophotometry to determine the existence of fluorescence in a given sample.
Chemical markers
UV fluorescent dyes are used in many applications (for example, biochemistry and forensics). The Green Fluorescent Protein (GFP) is often used in genetics as a marker. Many substances, such as proteins, have significant light absorption bands in the ultraviolet that are of use and interest in biochemistry and related fields. UV-capable spectrophotometers are common in such laboratories.
Photochemotherapy
Exposure to UVA light while the skin is hyper-photosensitive by taking psoralens is an effective treatment for psoriasis called PUVA. Due to psoralens potentially causing damage to the liver, PUVA may only be used a limited number of times over a patient's lifetime.
Phototherapy
Exposure to UVB light, particularly the 310 nm narrowband UVB range, is an effective long-term treatment for many skin conditions like psoriasis, vitiligo, eczema, and many others. UVB phototherapy does not require additional medications or topical preparations for the therapeutic benefit; only the light exposure is needed. However, phototherapy can be effective when used in conjunction with certain topical treatments such as anthralin, coal tar, and Vitamin A and D derivatives, or systemic treatments such as methotrexate and soriatane.[15]
Typical treatment regimes involve short exposure to UVB rays 3 to 5 times a week at a hospital or clinic, and for the best results, up to 30 or more sessions may be required.
Side effects may include itching and redness of the skin due to UVB exposure, and possibly sunburn, if patients do not minimize exposure to natural UV rays during treatment days.
Sterilization

Ultraviolet lamps are used to sterilize workspaces and tools used in biology laboratories and medical facilities. Commercially-available low pressure mercury-vapor lamps emit about 86% of their light at 254 nanometers (nm) which coincides very well with one of the two peaks of the germicidal effectiveness curve (i.e., effectiveness for UV absorption by DNA). One of these peaks is at about 265 nm and the other is at about 185 nm. Although 185 nm is better absorbed by DNA, the quartz glass used in commercially-available lamps, as well as environmental media such as water, are more opaque to 185 nm than 254 nm (C. von Sonntag et al., 1992). UV light at these germicidal wavelengths causes adjacent thymine molecules on DNA to dimerize, if enough of these defects accumulate on a microorganism's DNA its replication is inhibited, thereby rendering it harmless (even though the organism may not be killed outright). However, since microorganisms can be shielded from ultraviolet light in small cracks and other shaded areas, these lamps are used only as a supplement to other sterilization techniques.
Disinfecting drinking water
UV radiation can be an effective viricide and bactericide. Disinfection using UV radiation is commonly used in wastewater treatment applications and is finding an increased usage in drinking water treatment. Many bottlers of spring water use UV disinfection equipment to sterilize their water.
New York City has approved the construction of a 2 billion gallon per day ultraviolet drinking water disinfection facility[16]. There are also several facilities under construction and several in operation that treat waste water with several stages of filters, hydrogen peroxide and UV light to bring the water up to drinking standards. One such facility exists in Orange County California. [17] [18]
It used to be thought that UV disinfection was more effective for bacteria and viruses, which have more exposed genetic material, than for larger pathogens which have outer coatings or that form cyst states (e.g., Giardia) that shield their DNA from the UV light. However, it was recently discovered that ultraviolet radiation can be somewhat effective for treating the microorganism Cryptosporidium. The findings resulted in two US patents and the use of UV radiation as a viable method to treat drinking water. Giardia in turn has been shown to be very susceptible to UV-C when the tests were based on infectivity rather than excystation.[19] It has been found that protists are able to survive high UV-C doses but are sterilized at low doses.
A process named SODIS [2] has been extensively researched in Switzerland and has proven ideal to treat small quantities of water using natural sunlight. Contaminated water is poured into transparent plastic bottles and exposed to full sunlight for six hours. The sunlight treats the contaminated water through two synergetic mechanisms: Radiation in the spectrum of UV-A (wavelength 320-400 nm) and increased water temperature. If the water temperatures rises above 50 °C, the disinfection process is three times faster.
Food processing
As consumer demand for fresh and "fresh-like" food products increases, the demand for nonthermal methods of food processing is likewise on the rise. In addition, public awareness regarding the dangers of food poisoning is also raising demand for improved food processing methods. Ultraviolet radiation is used in several food processes to kill unwanted microorganisms. UV light can be used to pasteurize fruit juices by flowing the juice over a high intensity ultraviolet light source. The effectiveness of such a process depends on the UV absorbance of the juice (Beer's law).
Sun Tanning
Sun tanning describes a darkening of the skin (especially of fair-skinned individuals) in a natural physiological response stimulated by exposure to ultraviolet radiation from sunshine (or a sunbed). With excess exposure to the sun, a suntanned area can also develop sunburn.
Lasers
Ultraviolet lasers have applications in industry (laser engraving), medicine (dermatology and keratectomy), free air secure communications and computing (optical storage). They can be made by applying frequency conversion to lower-frequency lasers, or from Ce:LiSAF crystals (cerium doped with lithium strontium aluminum fluoride), a process developed in the 1990s at Lawrence Livermore National Laboratory.[20]
Evolutionary significance
Evolution of early reproductive proteins and enzymes is attributed in modern models of evolutionary theory to ultraviolet light. Ultraviolet light causes thymine base pairs next to each other in genetic sequences to bond together into thymine dimers, a disruption in the strand which reproductive enzymes cannot copy (see picture above). This leads to frameshifting during genetic replication and protein synthesis, usually killing the organism. As early prokaryotes began to approach the surface of the ancient oceans, before the protective ozone layer had formed, blocking out most wavelengths of UV light, they almost invariably died out. The few that survived had developed enzymes which verified the genetic material and broke up thymine dimer bonds, known as excision repair enzymes. Many enzymes and proteins involved in modern mitosis and meiosis are extremely similar to excision repair enzymes, and are believed to be evolved modifications of the enzymes originally used to overcome UV light.[21]
See also
- UV index
- High-energy visible light
- Sun tanning
- Black light
- Wood's lamp
- Tanning lamp
- UV Stabilizers in plastics
- Ultraviolet photography
Further reading
- Hu, S; Ma, F; Collado-Mesa, F; Kirsner, R. S. (2004), "UV radiation, latitude, and melanoma in US Hispanics and blacks", Arch. Dermatol., 140 (7): 819–824, doi:10.1001/archderm.140.7.819 Unknown parameter
|month=ignored (help) - Hockberger, Philip E. (2002), "A History of Ultraviolet Photobiology for Humans, Animals and Microorganisms" (PDF), Photochemisty and Photobiology, 76 (6): 561–569,Error: Bad DOI specified!
- Allen, Jeannie (2001-09-06), Ultraviolet Radiation: How it Affects Life on Earth, Earth Observatory, NASA, USA
References
- ↑ Hockberger, P. E. (2002), "A history of ultraviolet photobiology for humans, animals and microorganisms", Photochem. Photobiol., 76: 561–579
- ↑ "ISO 21348 Process for Determining Solar Irradiances".
- ↑ "Soda Lime Glass Transmission Curve".
- ↑ "B270-Superwite Glass Transmission Curve".
- ↑ "Selected Float Glass Transmission Curve".
- ↑ Template:Cite paper
- ↑ 7.0 7.1 The Science of Sun Protection, Talk of the Nation Science Friday, 24 June 2005. Vitamin D pills recommended over sun exposure, but most people in Australia and Canada get enough Vitamin D by incidental exposure, studies show.
- ↑ "Health effects of UV radiation".
- ↑ Davies H.; Bignell G. R.; Cox C.; (2002). "Mutations of the BRAF gene in human cancer". Nature. 417: 949–954. Unknown parameter
|month=ignored (help) - ↑ Torma, H; Berne, B; Vahlquist, A (1988), "UV irradiation and topical vitamin A modulate retinol esterification in hairless mouse epidermis", Acta Derm. Venereol., 68 (4): 291--299
- ↑ Autier P; Dore J F; Schifflers E; et al. (1995). "Melanoma and use of sunscreens: An EORTC case control study in Germany, Belgium and France". Int. J. Cancer. 61: 749–755.
- ↑ Hanson Kerry M.; Gratton Enrico; Bardeen Christopher J. (2006). "Sunscreen enhancement of UV-induced reactive oxygen species in the skin". Free Radical Biology and Medicine. 41 (8): 1205–1212.
- ↑ Template:Cite paper
- ↑ Template:Cite paper
- ↑ "UVB Phototherapy". National Psoriasis Foundation, USA. Archived from the original (php) on 2007-06-22. Retrieved 2007-09-23.
- ↑ Template:Cite paper
- ↑ http://www.latimes.com/news/local/la-me-reclaim2jan02,0,7789563.story?coll=la-home-center
- ↑ New Purification Plant Answers California's Water Crisis
- ↑ Template:Cite paper
- ↑ Marshall, Chris (1996). "A simple, reliable ultraviolet laser: the Ce:LiSAF". Lawrence Livermore National Laboratory. Retrieved 2008-01-11.
- ↑ Template:Cite paper
ar:أشعة فوق بنفسجية bg:Ултравиолетови лъчи br:Uslimestra bs:Ultraljubičasto zračenje ca:Ultraviolat cs:Ultrafialové záření da:Ultraviolet lys de:Ultraviolettstrahlung eo:Ultraviola radiado et:Ultraviolettkiirgus eu:Ultramore fa:فرابنفش fi:Ultraviolettisäteily gl:Ultravioleta he:על-סגול hi:पराबैंगनी hr:Ultraljubičasto zračenje id:Ultraungu io:Ultreviolea is:Útfjólublátt ljós it:Radiazione ultravioletta kn:ಅತಿನೇರಳೆ ವಿಕಿರಣ ko:자외선 ksh:Shwatzleesh lt:Ultravioletiniai spinduliai lv:Ultravioletais starojums hu:Ultraibolya sugárzás mk:Ултравиолетова светлина ms:Ultraungu nds:Ultravigelett nl:Ultraviolet no:Ultrafiolett stråling om:Ultraviolet sh:Ultraljubičasto zračenje simple:Ultraviolet sk:Ultrafialové žiarenie sl:Ultravijolično valovanje sq:Rrezet ultravioletë sr:Ултраљубичасто зрачење sv:Ultraviolett strålning ta:புற ஊதா கதிர் th:รังสีอัลตราไวโอเลต uk:Випромінювання ультрафіолетове zh-min-nan:Chí-goā-soàⁿ