Ticarcillin clavulanate description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Description
TIMENTIN (ticarcillin disodium and clavulanate potassium) for Injection, 3.1‑gram glass vial, 31‑gram Pharmacy Bulk Package, and TIMENTIN (ticarcillin disodium and clavulanate potassium) Injection in the GALAXY Container (PL 2040 Plastic) are a combination of ticarcillin disodium and the β‑lactamase inhibitor clavulanate potassium (the potassium salt of clavulanic acid) for intravenous administration. Ticarcillin is derived from the basic penicillin nucleus, 6‑amino‑penicillanic acid.
Chemically, ticarcillin disodium is N-(2-Carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-6-yl)-3-thiophenemalonamic acid disodium salt and may be represented as:
Clavulanic acid is produced by the fermentation of Streptomyces clavuligerus. It is a β‑lactam structurally related to the penicillins and possesses the ability to inactivate a wide variety of β‑lactamases by blocking the active sites of these enzymes. Clavulanic acid is particularly active against the clinically important plasmid‑mediated β‑lactamases frequently responsible for transferred drug resistance to penicillins and cephalosporins.
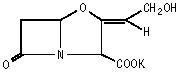
Chemically, clavulanate potassium is potassium (Z)-(2R,5R)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-azabicyclo[3.2.0]heptane-2-carboxylate and may be represented structurally as:
TIMENTIN (ticarcillin disodium and clavulanate potassium) for Injection, the 3.1‑gram glass vial or the 31‑gram Pharmacy Bulk Package, are white to pale yellow sterile powders to be reconstituted and diluted for intravenous infusion. The reconstituted solution is clear, colorless or pale yellow, with a pH of 5.5 to 7.5. The 3.1‑gram glass vial of TIMENTIN for Injection contains ticarcillin disodium equivalent to 3 grams ticarcillin and clavulanate potassium equivalent to 0.1 gram clavulanic acid. The 31‑gram TIMENTIN for Injection Pharmacy Bulk Package contains ticarcillin disodium equivalent to 30 grams ticarcillin and clavulanate potassium equivalent to 1 gram clavulanic acid.
TIMENTIN (ticarcillin disodium and clavulanate potassium) Injection in the GALAXY Container (PL 2040 Plastic) is an iso‑osmotic, sterile, nonpyrogenic, frozen solution containing 3.0 grams ticarcillin as ticarcillin disodium and 0.1 gram clavulanic acid as clavulanate potassium. Approximately 0.3 gram sodium citrate hydrous, USP, is added as a buffer. Sodium hydroxide is used to adjust pH and convert ticarcillin monosodium to ticarcillin disodium. The pH may have been adjusted with hydrochloric acid. The solution is intended for intravenous use after thawing to room temperature. The pH of thawed solution ranges from 5.5 to 7.5.
The GALAXY container is fabricated from a specially designed multilayer plastic, PL 2040. Solutions are in contact with the polyethylene layer of this container and can leach out certain chemical components of the plastic in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
For the 3.1-gram dosage of TIMENTIN, the theoretical sodium content is 4.51 mEq (103.6 mg) per gram of TIMENTIN. The theoretical potassium content is 0.15 mEq (6 mg) per gram of TIMENTIN.[1]
References
Adapted from the FDA Package Insert.