Sporotrichosis laboratory findings
|
Sporotrichosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sporotrichosis laboratory findings On the Web |
|
American Roentgen Ray Society Images of Sporotrichosis laboratory findings |
|
Risk calculators and risk factors for Sporotrichosis laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alison Leibowitz [2]
Overview
Laboratory findings consistent with the diagnosis of sporotrichosis include isolation of S. schenckii upon culture, molecular detection, a positive sporotrichin skin test, and techniques involving antibody detection. Definitive diagnosis of sporotrichosis occurs upon the isolation and identification of S. schenckii in culture.
Laboratory findings
- Sporotrichosis is a chronic disease with slow progression and often subtle symptoms. It is difficult to diagnose, as many other diseases share similar symptoms and therefore must be ruled out.
- While within human and animal tissues, S. schenckii exists in its yeast form.
- Varying in size and shape, these curved cells typically have 2-6 μm diameters with cigar-like buds offshooting from a narrow base.
- Growing on Sabouraud dextrose agar, most S. schenckii strains become evident after 4 days. At this point, some strains lack dark pigment, while others have been infiltrated with dark pigment from the start. Upon transfer to Brain-Heart Infusion (BHI) agar, and cultured for 7 days at 37°C, the S. schenckii strains undergo dimorphism, manifesting as creamy off-white to beige colored colonies.[1]
- Patients with sporotrichosis will likely have antibodies against the fungus S. schenckii, however, due to variability in sensitivity and specificity, antibody identification may not be a reliable diagnosis for this disease. The confirming diagnosis remains culturing the fungus from the skin, sputum, synovial fluid, and cerebrospinal fluid.
- Cats with sporotrichosis are unique in that the exudate from their lesions may contain numerous organisms. This makes cytological evaluation of exudate a valuable diagnostic tool in this species. Exudate is pyogranulomatous and phagocytic cells may be packed with yeast forms. These are variable in size, but many are cigar-shaped.[2]
Culture and Identification
- Definitive diagnosis of sporotrichosis occurs upon the isolation and identification of S. schenckii in culture.
- The etiological agent may be obtained when the specimen is cultured on Sabouraud agar with chloramphenicol or on mycobiotic agar.[3]
- Filamentous hyaline colonies begin to grow following 5-7 days of incubation at 25ºC, and may manifest with pigmented centers.[4]
- Ultimately, the isolation of S. schenckii is dependent on 5-7 day a subculture of the fungus on enriched agars, such as Brain Heart Infusion agar, at 35-37ºC. Isolation is verified following the demonstration of dimorphism.[1]
- In some cases, isolation requires multiple subcultures.
- While positive a culture is the strongest indication for diagnosis, this method may be more realistic in cutaneous forms of the disease than in certain systematic manifestations, due to greater lengths required for the collection of fungus in systematic forms.[1]
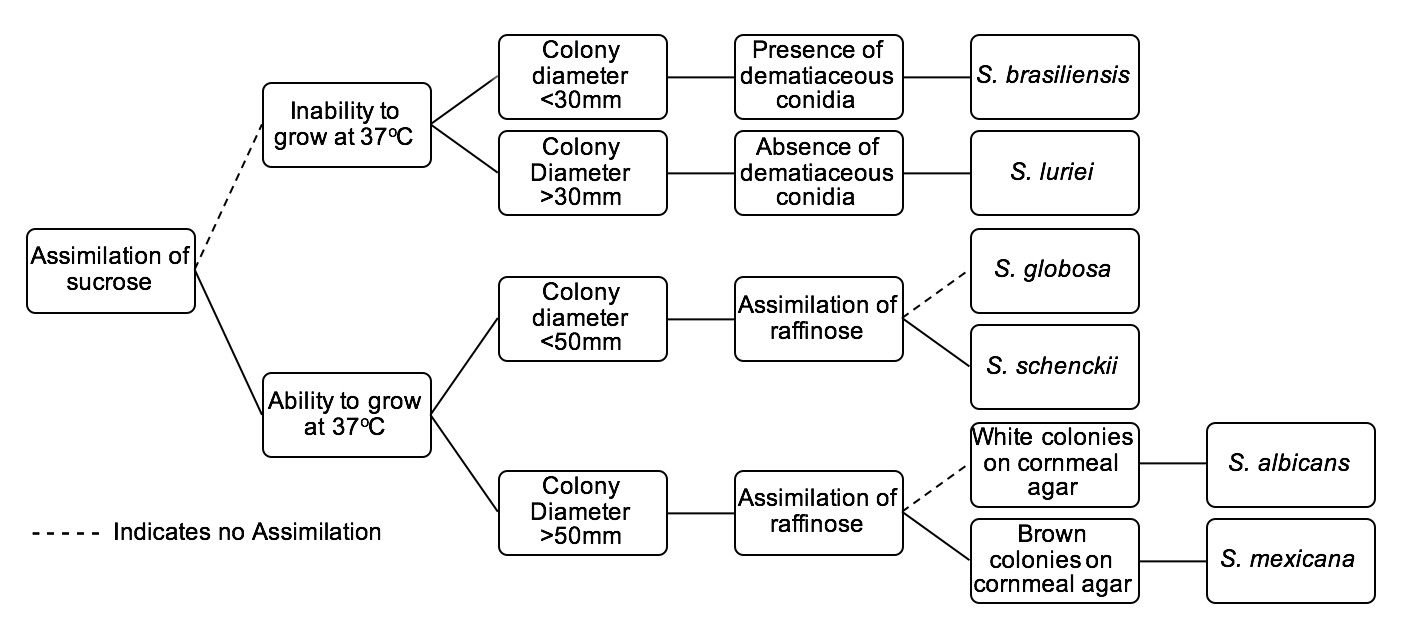
The gold standard for diagnosis of Sporotrichosis is with fungal culture. The tree below serves as an identification key for six Sporothrix species of clinical interest:
Molecular Detection and Identification
- Though there is a scarcity of molecular methods of diagnosis for Sporotrichosis in standard care, researchers have identified a variety of detection systems.
- Molecular findings specific to specimens of Sporotrichosis:
- Detected particular nucleic acid probes that target large subunit rRNA genes from S. schenckii
- Additionally, the researchers extracted DNA from clinical specimens by boiling them in an alkaline guanidine-phenol-Tris reagent, amplifying a segment of the 28S rRNA gene with universal primers, and utilizing amplicon identification via probe hybridization.[5]
Sporotrichin Skin Test
- While the cutaneous sporotrichin skin test can serve as a helpful diagnostic tool, it is mainly utilized in epidemiological studies.
- The test detects the cellular immune response, delayed hypersensitivity.
- In roughly 90% of confirmed sporotrichosis cases, the test comes back positive.[6]
- A positive test result may also result following a previous S. schenckii infection.
- There is a lack of standardization in regards to antigen production, which may result in a variation in findings.[1]
Antibody Detection and Identification
- Findings from antibody detection techniques merely establish speculative diagnosis for sporotrichosis. The use of additional methods of clinical and epidemiological associations is necessary for definite diagnosis.
- Antibody detection in sera from infected hosts has been outlined as a diagnostic tool for cases of sporotrichosis.
- Precipitation and agglutination methods were among the first techniques.
- Double immunodiffusion does not typically display cross reactions with the sera specimens taken from hosts with other infectious diseases that have similar clinical findings, namely chromoblastomycosis and leishmaniasis.
- Researchers have found that an anodic arc, or S arc, upon immunoelectrophoresis is characteristic of cases of sporotrichosis.[7]
- Tube and latex agglutination have been used since the 1970's as a means of serodiagnosis, though this technique is less effective in cases of cutaneous sporotrichosis.
- Immunoenzymatic assays have been used as a means of serodiagnosis.
- An enzyme-linked immunosorbent assay (ELISA) was developed for specific antibody detection in serum specimens of patients with sporotrichosis.[8]
- In 2007, researchers discussed an enzyme-linked immunosorbent assay, also known as ELISA. ELISA functions by detecting particular antibodies in the serum samples from infected patients. Though ELISA demonstrated 100% sensitivity to all 35 positive serum samples, sera samples from patients with cutaneous leishmaniasis resulted in cross-results.
- In instances involving cross-results and false positives, ELISA may be use in addition to other diagnostic techniques.
- Exoantigens formed by a mycelial-phase S. schenckii strain were isolated amidst an epidemic in Rio de Janeiro, Brazil. This epidemic was the first that was rooted in zoonotic transmission.
- Researchers developed an enzyme immunoassay with these exoantigens.
- Researchers described and experimented with this antigen, finding no cross-results with sera taken from patients with other mycoses.[9]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Barros MB, de Almeida Paes R, Schubach AO (2011). "Sporothrix schenckii and Sporotrichosis". Clin Microbiol Rev. 24 (4): 633–54. doi:10.1128/CMR.00007-11. PMC 3194828. PMID 21976602.
- ↑ Alvarado-Ramírez E, Torres-Rodríguez JM (2007). "In vitro susceptibility of Sporothrix schenckii to six antifungal agents determined using three different methods". Antimicrob Agents Chemother. 51 (7): 2420–3. doi:10.1128/AAC.01176-06. PMC 1913275. PMID 17438048.
- ↑ Oliveira DC, Lopes PG, Spader TB, Mahl CD, Tronco-Alves GR, Lara VM; et al. (2011). "Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil". J Clin Microbiol. 49 (8): 3047–9. doi:10.1128/JCM.00255-11. PMC 3147739. PMID 21653757.
- ↑ Morris-Jones R (2002). "Sporotrichosis". Clin Exp Dermatol. 27 (6): 427–31. PMID 12372075.
- ↑ Sandhu GS, Kline BC, Stockman L, Roberts GD (1995). "Molecular probes for diagnosis of fungal infections". J Clin Microbiol. 33 (11): 2913–9. PMC 228606. PMID 8576345.
- ↑ Itoh M, Okamoto S, Kariya H (1986). "Survey of 200 cases of sporotrichosis". Dermatologica. 172 (4): 209–13. PMID 3709907.
- ↑ de Albornoz MB, Villanueva E, de Torres ED (1984). "Application of immunoprecipitation techniques to the diagnosis of cutaneous and extracutaneous forms of sporotrichosis". Mycopathologia. 85 (3): 177–83. PMID 6429540.
- ↑ Almeida-Paes R, Pimenta MA, Pizzini CV, Monteiro PC, Peralta JM, Nosanchuk JD; et al. (2007). "Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection". Clin Vaccine Immunol. 14 (3): 244–9. doi:10.1128/CVI.00430-06. PMC 1828849. PMID 17215334.
- ↑ Mendoza M, Díaz AM, Hung MB, Zambrano EA, Díaz E, De Albornoz MC (2002). "Production of culture filtrates of Sporothrix schenckii in diverse culture media". Med Mycol. 40 (5): 447–54. PMID 12462523.