Rifampin labels and packages
Jump to navigation
Jump to search
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Labels And Packages
PRINCIPAL DISPLAY PANEL - 150mg Bottle Label
NDC 0068-0510-30 Rifadin® 150mg rifampin capsules USP 30 Capsules sanofi aventis
 |
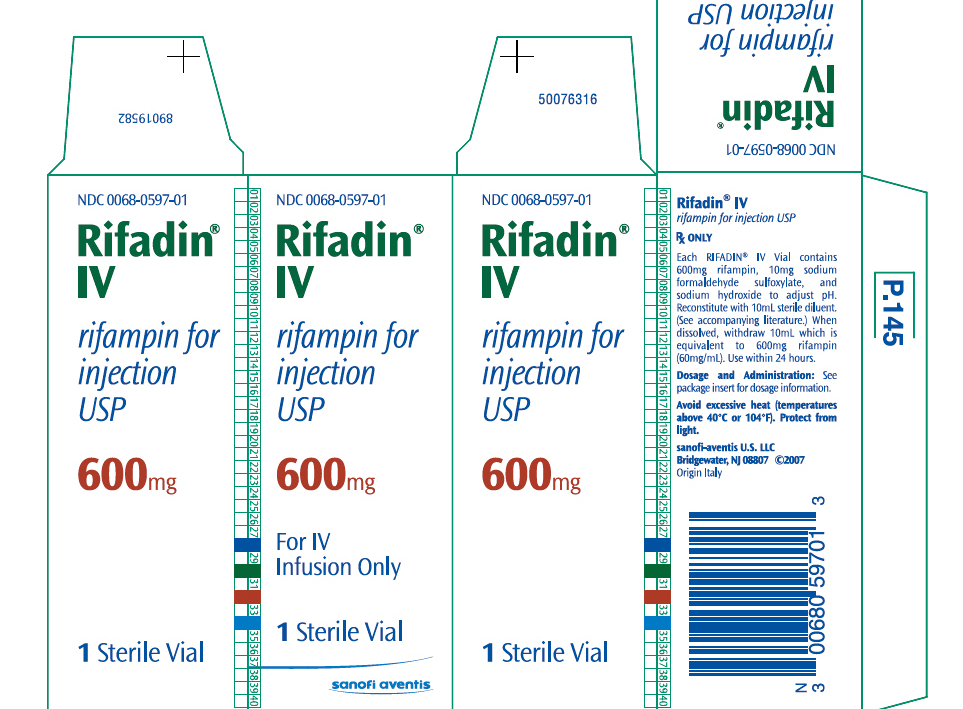
PRINCIPAL DISPLAY PANEL - 600mg Vial Carton
NDC 0068-0597-01 Rifadin® IV rifampin for injection USP 600mg For IV Infusion Only 1 Sterile Vial sanofi aventis [1]
 |
References
Adapted from the FDA Package Insert.