Piperacillin tazobactam sodium microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Microbiology
Mechanism of Action
Piperacillin sodium exerts bactericidal activity by inhibiting septum formation and cell wall synthesis of susceptible bacteria. In vitro, piperacillin is active against a variety of Gram-positive and Gram-negative aerobic and anaerobic bacteria. Tazobactam sodium has little clinically relevant in vitro activity against bacteria due to its reduced affinity to penicillin-binding proteins. It is, however, a β-lactamase inhibitor of the Molecular class A enzymes, including Richmond-Sykes class III (Bush class 2b & 2b') penicillinases and cephalosporinases. It varies in its ability to inhibit class II and IV (2a & 4) penicillinases. Tazobactam does not induce chromosomally-mediated β-lactamases at tazobactam concentrations achieved with the recommended dosage regimen.
Spectrum of Activity
Piperacillin/tazobactam has been shown to be active against most isolates of the following microorganisms both in vitro and in clinical infections.
Gram-positive bacteria:
- Staphylococcus aureus (methicillin susceptible isolates only)
Gram-negative bacteria:
- Acinetobacter baumannii
- Escherichia coli
- Haemophilus influenzae (excluding β-lactamase negative, ampicillin-resistant isolates)
- Klebsiella pneumoniae
- Pseudomonas aeruginosa (given in combination with an aminoglycoside to which the isolate is susceptible)
Anaerobic bacteria:
- Bacteroides fragilis group (B. fragilis, B. ovatus, B. thetaiotaomicron, and B. vulgatus)
The following in vitro data are available, but their clinical significance is unknown.
At least 90% of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for piperacillin/tazobactam. However, the safety and effectiveness of piperacillin/tazobactam in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-positive bacteria:
- Enterococcus faecalis (ampicillin or penicillin-susceptible isolates only)
- [[Staphylococcus epidermidis (methicillin susceptible isolates only)
- Streptococcus agalactiae2
- Streptococcus pneumoniae2 (penicillin-susceptible isolates only)
- Streptococcus pyogenes2
- Viridans group streptococci2
Gram-negative bacteria:
- Citrobacter koseri
- Moraxella catarrhalis
- Morganella morganii
- Neisseria gonorrhoeae
- Proteus mirabilis
- Proteus vulgaris
- Serratia marcescens
- Providencia stuartii
- Providencia rettgeri
- Salmonella enterica
Anaerobic bacteria:
- Clostridium perfringens
- Bacteroides distasonis
- Prevotella melaninogenica
2 These are not β-lactamase producing bacteria and, therefore, are susceptible to piperacillin alone.
Susceptibility Testing Methods
As is recommended with all antimicrobials, the results of in vitro susceptibility tests, when available, should be provided to the physician as periodic reports, which describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting the most effective antimicrobial.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of piperacillin and tazobactam powders.1,2 MIC values should be determined using serial dilutions of piperacillin combined with a fixed concentration of 4 μg/mL tazobactam. The MIC values obtained should be interpreted according to criteria provided in Table 8.
Diffusion Technique
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method1,3 and requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 100 mcg of piperacillin and 10 mcg of tazobactam to test the susceptibility of microorganisms to piperacillin/tazobactam. The disk diffusion interpreted criteria are provided in Table 8.
Anaerobic Techniques
For anaerobic bacteria, the susceptibility to piperacillin/tazobactam can be determined by the reference agar dilution method.4
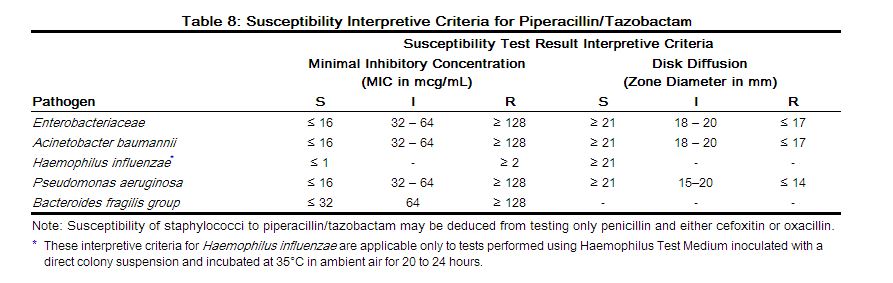 |
A report of S ("Susceptible") indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentration at the infection site necessary to inhibit growth of the pathogen. A report of I ("Intermediate") indicates that the results should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone, which prevents small, uncontrolled technical factors from causing major discrepancies in interpretation. A report of R ("Resistant") indicates that the pathogen is not likely to be inhibited even if the antimicrobial compound in the blood reaches the concentration usually achievable at the infection site; other therapy should be considered.
Quality Control
Standardized susceptibility test procedures require the use of quality controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test procedures.1,2,3,4 Standard piperacillin/tazobactam powder should provide the following ranges of values noted in Table 9. Quality control bacteria are specific strains of bacteria with intrinsic biological properties relating to resistance mechanisms and their genetic expression within the microorganism; the specific strains used for microbiological quality control are not clinically significant.[1]
 |
References
- ↑ "ZOSYN (PIPERACILLIN SODIUM AND TAZOBACTAM SODIUM) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION ZOSYN PHARMACY BULK PACKAGE (PIPERACILLIN SODIUM AND TAZOBACTAM SODIUM) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION ZOSYN IN GALAXY CONTAINERS (TAZOBACTAM SODIUM AND PIPERACILLIN SODIUM) INJECTION, SOLUTION [WYETH PHARMACEUTICALS INC., A SUBSIDIARY OF PFIZER INC.]".
Adapted from the FDA Package Insert.