Penicillin G potassium dosage and administration
| Penicillin G potassium |
|---|
| PENICILLIN G POTASSIUM® FDA Package Insert |
| Description |
| Clinical Pharmacology |
| Microbiology |
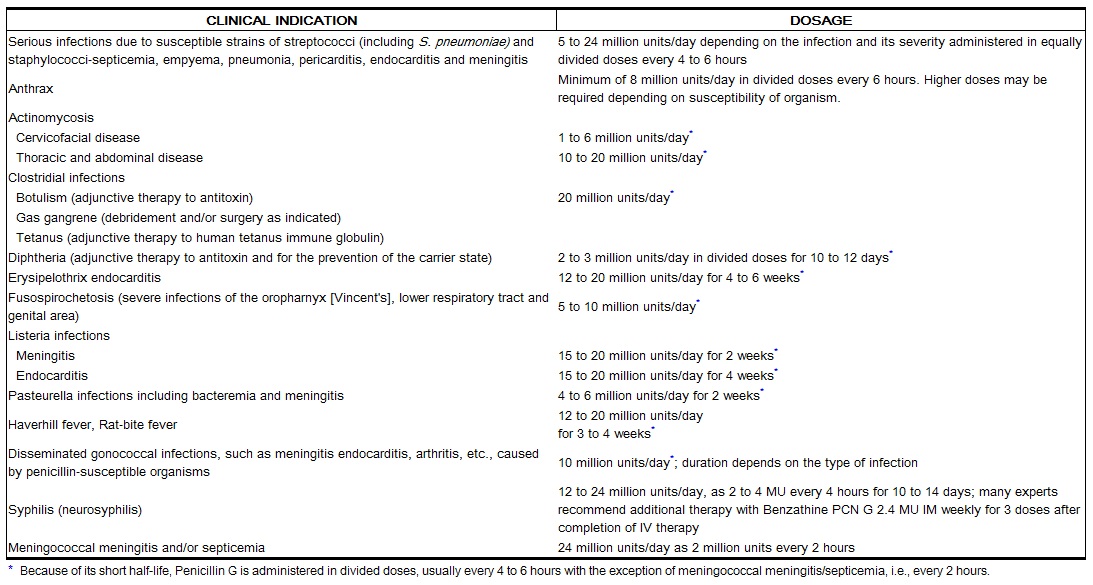
| Indications and Usage |
| Contraindications |
| Warnings and Precautions |
| Adverse Reactions |
| Overdosage |
| Dosage and Administration |
| How Supplied |
| Labels and Packages |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Dosage and Administration
Buffered Penicillin G Potassium for Injection, USP may be given intravenously or intramuscularly. The usual dose recommendations are as follows:
Pediatric patients
This product should not be administered to patients requiring less than one million units per dose. (see PRECAUTIONS – Pediatric Use).
Renal Impairment
Penicillin G is relatively nontoxic, and dosage adjustments are generally required only in cases of severe renal impairment.
The recommended dosage regimens are as follows: Creatinine clearance less than 10 mL/min/1.73m2; administer a full loading dose (see recommended dosages in the tables above) followed by one-half of the loading dose every 8 to 10 hours.
Uremic patients with a creatinine clearance greater than 10 mL/min/1.73m2; administer a full loading dose (see recommended dosages in the tables above) followed by one-half of the loading dose every 4 to 5 hours.
Additional dosage modifications should be made in patients with hepatic disease and renal impairment.
For most acute infections, treatment should be continued for at least 48 to 72 hours after the patient becomes asymptomatic. Antibiotic therapy for Group A β-hemolytic streptococcal infections should be maintained for at least 10 days to reduce the risk of rheumatic fever.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Preparation of Solution
Solutions of penicillin should be prepared as follows: Loosen powder. Hold vial horizontally and rotate it while slowly directing the stream of diluent against the wall of the vial. Shake vial vigorously after all the diluent has been added. Depending on the route of administration, use Sterile Water for Injection, USP or Sterile Isotonic Sodium Chloride Solution for Parenteral use.
Note: Penicillins are rapidly inactivated in the presence of carbohydrate solutions at alkaline pH.
Reconstitution
The following table shows the amount of solvent required for solution of various concentrations:

When the required volume of solvent is greater than the capacity of the vial, the penicillin can be dissolved by first injecting only a portion of the solvent into the vial, then withdrawing the resultant solution and combining it with the remainder of the solvent in a larger sterile container.
Penicillin G Potassium for Injection, USP is highly water soluble. It may be dissolved in small amounts of Water for Injection, or Sterile Isotonic Sodium Chloride Solution for Parenteral Use. All solutions should be stored in a refrigerator. When refrigerated, pencillin solutions may be stored for seven days without significant loss of potency.
Buffered Penicillin G Potassium for Injection may be given intramuscularly or by continuous intravenous drip for dosages of 500,000, 1,000,000 or 5,000,000 units. It is also suitable for intrapleural, intraarticular, and other local installations.
THE 20,000,000 UNIT (20 MILLION UNIT) DOSAGE MAY BE ADMINISTERED BY INTRAVENOUS INFUSION ONLY.
(1) Intramuscular Injection: Keep total volume of injection small. The intramuscular route is the preferred route of administration. Solutions containing up to 100,000 units of penicillin per mL of diluent may be used with a minimum of discomfort. Greater concentration of penicillin G per mL is physically possible and may be employed where therapy demands. When large doses are required, it may be advisable to administer aqueous solutions of penicillin by means of continuous intravenous drip.
(2) Continuous Intravenous Drip: Determine the volume of fluid and rate of its administration required by the patient in a 24-hour period in the usual manner for fluid therapy, and add the appropriate daily dosage of penicillin to this fluid. For example, if an adult patient requires 2 liters of fluid in 24 hours and a daily dosage of 10 million units of penicillin, add 5 million units of 1 liter and adjust the rate of flow so the liter will be infused in 12 hours.
(3) Intrapleural or Other Local Infusion: If fluid is aspirated, give infusion in a volume equal to 1/4 or 1/2 the amount of fluid aspirated, otherwise, prepare as for an intramuscular injection.
(4) Intrathecal Use: The intrathecal use of penicillin in meningitis must be highly individualized. It should be employed only with full consideration of the possible irritating effects of penicillin when used by this route. The preferred route of therapy in bacterial meningitides is intravenous, supplemented by intramuscular injection.[1]
References
- ↑ "PENICILLIN G POTASSIUM INJECTION, POWDER, FOR SOLUTION [SANDOZ INC]". Text " accessdate" ignored (help)
Adapted from the FDA Package Insert.
References
Adapted from the FDA Package Insert.
