Ethotoin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ethotoin is an anticonvulsant that is FDA approved for the treatment of tonic-clonic (grand mal) and complex partial (psychomotor) seizures. Common adverse reactions include stevens-johnson syndrome, disorder of hematopoietic structure, megaloblastic anemia, lymphadenopathy, systemic lupus erythematosus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Ethotoin is indicated for the control of tonic-clonic (grand mal) and complex partial (psychomotor) seizures.
- Ethotoin is administered orally in 4 to 6 divided doses daily. The drug should be taken after food, and doses should be spaced as evenly as practicable. Initial dosage should be conservative. For adults, the initial daily dose should be 1 g or less, with subsequent gradual dosage increases over a period of several days. The optimum dosage must be determined on the basis of individual response. The usual adult maintenance dose is 2 to 3 g daily. Less than 2 g daily has been found ineffective in most adults.
- Pediatric dosage depends upon the age and weight of the patient. The initial dose should not exceed 750 mg daily. The usual maintenance dose in children ranges from 500 mg to 1 g daily, although occasionally 2 or (rarely) 3 g daily may be necessary.
- If a patient is receiving another antiepileptic drug, it should not be discontinued when PEGANONE therapy is begun. The dosage of the other drug should be reduced gradually as that of PEGANONE is increased. PEGANONE may eventually replace the other drug or the optimal dosage of both antiepileptics may be established.
- In tonic-clonic (grand mal) seizures, use of the drug with phenobarbital may be beneficial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ethotoin in adult patients.
Non–Guideline-Supported Use
=
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ethotoin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Ethotoin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ethotoin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ethotoin in pediatric patients.
Contraindications
- Ethotoin is contraindicated in patients with hepatic abnormalities or hematologic disorders.
Warnings
- Suicidal Behavior and Ideation: Antiepileptic drugs (AEDs), including PEGANONE, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. TABLE 1 shows absolute and relative risk by indication for all evaluated AEDs.
- The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
- Anyone considering prescribing PEGANONE or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness.
- Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
- Use in Pregnancy: ethotoin can cause fetal harm when administered to a pregnant woman. There are multiple reports in the clinical literature which indicate that the use of antiepileptic drugs during pregnancy results in an increased incidence of birth defects in the offspring. Although data are more extensive with respect to phenytoin and phenobarbital, reports indicate a possible similar association with the use of other antiepileptic drugs. Therefore, antiepileptic drugs should be administered to women of child-bearing potential only if they are clearly shown to be essential in the management of their seizures.
- Antiepileptic drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and risk to both mother and the unborn child. Consideration should, however, be given to discontinuation of antiepileptics prior to and during pregnancy when the nature, frequency and severity of the seizures do not pose a serious threat to the patient. It is not, however, known whether even minor seizures constitute some risk to the developing embryo or fetus.
- Reports have suggested that the maternal ingestion of antiepileptic drugs, particularly barbiturates, is associated with a neonatal coagulation defect that may cause bleeding during the early (usually within 24 hours of birth) neonatal period. The possibility of the occurrence of this defect with the use of PEGANONE should be kept in mind. The defect is characterized by decreased levels of vitamin k-dependent clotting factors, and prolongation of either the prothrombin time or the partial thromboplastin time, or both. It has been suggested that vitamin k be given prophylactically to the mother one month prior to and during delivery, and the infant, intravenously, immediately after birth.
- If PEGANONE is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- To provide information regarding the effects of in utero exposure to PEGANONE, physicians are advised to recommend that pregnant patients taking PEGANONE enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll-free number 1-888-233-2334, and must be done by patients themselves.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Ethotoin in the drug label.
Postmarketing Experience
- Adverse reactions associated with PEGANONE, in decreasing order of severity, are:
- Isolated cases of lymphadenopathy and systemic lupus erythematosus have been reported in patients taking hydantoin compounds, and lymphadenopathy has occurred with PEGANONE. Withdrawal of therapy has resulted in remission of the clinical and pathological findings. Therefore, if a lymphoma-like syndrome develops, the drug should be withdrawn and the patient should be closely observed for regression of signs and symptoms before treatment is resumed.
- Ataxia and gum hypertrophy have occurred only rarely—usually only in patients receiving an additional hydantoin derivative. It is of interest to note that ataxia and gum hypertrophy have subsided in patients receiving other hydantoins when ethotoin was given as a substitute antiepileptic.
- Occasionally, vomiting or nausea after ingestion of PEGANONE has been reported, but if the drug is administered after meals, the incidence of gastric distress is reduced. Other side effects have included chest pain, nystagmus, diplopia, fever, dizziness, diarrhea, headache, insomnia, fatigue, numbness, skin rash, and Stevens-Johnson syndrome.
Drug Interactions
There is limited information regarding Ethotoin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- ethotoin can cause fetal harm when administered to a pregnant woman. There are multiple reports in the clinical literature which indicate that the use of antiepileptic drugs during pregnancy results in an increased incidence of birth defects in the offspring. Although data are more extensive with respect to phenytoin and phenobarbital, reports indicate a possible similar association with the use of other antiepileptic drugs.
- Therefore, antiepileptic drugs should be administered to women of child-bearing potential only if they are clearly shown to be essential in the management of their seizures.
- Antiepileptic drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures because of the strong possibility of precipitating status epilepticus with attendant hypoxia and risk to both mother and the unborn child. Consideration should, however, be given to discontinuation of antiepileptics prior to and during pregnancy when the nature, frequency and severity of the seizures do not pose a serious threat to the patient. It is not, however, known whether even minor seizures constitute some risk to the developing embryo or fetus.
- Reports have suggested that the maternal ingestion of antiepileptic drugs, particularly barbiturates, is associated with a neonatal coagulation defect that may cause bleeding during the early (usually within 24 hours of birth) neonatal period. The possibility of the occurrence of this defect with the use of PEGANONE should be kept in mind. The defect is characterized by decreased levels of vitamin k-dependent clotting factors, and prolongation of either the prothrombin time or the partial thromboplastin time, or both. It has been suggested that vitamin k be given prophylactically to the mother one month prior to and during delivery, and the infant, intravenously, immediately after birth.
- If PEGANONE is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Reports have suggested that the maternal ingestion of antiepileptic drugs, particularly barbiturates, is associated with a neonatal coagulation defect that may cause bleeding during the early (usually within 24 hours of birth) neonatal period. The possibility of the occurrence of this defect with the use of PEGANONE should be kept in mind.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ethotoin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ethotoin during labor and delivery.
Nursing Mothers
- Ethotoin is excreted in breast milk. Because of the potential for serious adverse reactions in nursing infants from ethotoin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Ethotoin with respect to pediatric patients.
Geriatic Use
- Clinical studies of PEGANONE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Ethotoin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ethotoin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ethotoin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ethotoin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ethotoin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ethotoin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
- Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
IV Compatibility
There is limited information regarding IV Compatibility of Ethotoin in the drug label.
Overdosage
- Symptoms of acute overdosage include drowsiness, visual disturbance, nausea and ataxia. Coma is possible at very high dosage.
- Treatment should be begun by inducing emesis; gastric lavage may be considered as an alternative. General supportive measures will be necessary. A careful evaluation of blood-forming organs should be made following recovery.
Pharmacology
| |
| |
Ethotoin
| |
| Systematic (IUPAC) name | |
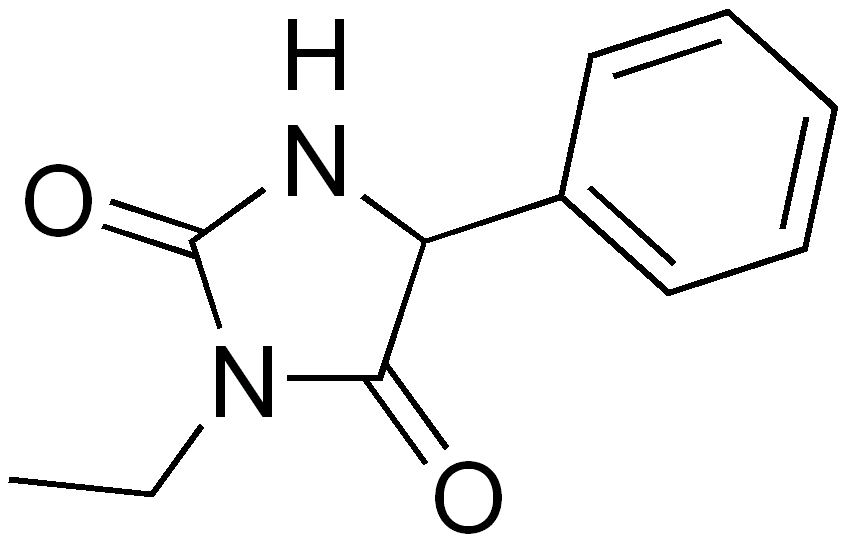
| 3-ethyl-5-phenyl-imidazolidine-2,4-dione | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 204.225 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 3 to 9 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Ethotoin exerts an antiepileptic effect without causing general central nervous system depression. The mechanism of action is probably very similar to that of phenytoin. The latter drug appears to stabilize rather than to raise the normal seizure threshold, and to prevent the spread of seizure activity rather than to abolish the primary focus of seizure discharges.
Structure
- Ethotoin is an oral antiepileptic of the hydantoin series and is chemically identified as 3-ethyl-5-phenyl-2,4-imidazolidinedione. It is represented by the following structural formula:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ethotoin in the drug label.
Pharmacokinetics
- Ethotoin exerts an antiepileptic effect without causing general central nervous system depression. The mechanism of action is probably very similar to that of phenytoin. The latter drug appears to stabilize rather than to raise the normal seizure threshold, and to prevent the spread of seizure activity rather than to abolish the primary focus of seizure discharges.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ethotoin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Ethotoin in the drug label.
How Supplied
- Ethotoin 250 mg grooved, white tablets bearing the letters OV on one side and the number 61 on the other and are supplied in bottles of 100 (NDC 55292-601-01).
Storage
Recommended storage: Store at 20-25ºC (68-77ºF).
Images
Drug Images
{{#ask: Page Name::Ethotoin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ethotoin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Read this Medication Guide before you start taking PEGANONE and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
- What is the most important information I should know about PEGANONE?
- Do not stop taking PEGANONE without first talking to your healthcare provider.
- Stopping PEGANONE suddenly can cause serious problems.
- PEGANONE can cause serious side effects, including:
- Like other antiepileptic drugs, PEGANONE may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
- Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- Thoughts about suicide or dying
- Attempts to commit suicide
- New or worse depression
- New or worse anxiety
- Feeling agitated or restless
- Panic attacks
- Trouble sleeping (insomnia)
- New or worse irritability
- Acting aggressive, being angry, or violent
- Acting on dangerous impulses
- An extreme increase in activity and talking (mania)
- Other unusual changes in behavior or mood
- How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
- Do not stop PEGANONE without first talking to a healthcare provider.
- Stopping PEGANONE suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
- Suicidal thoughts or actions can be caused by things other than medicines. * If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
- What is PEGANONE?
- PEGANONE is a prescription medicine used to treat tonic-clonic (grand mal) and complex partial (psychomotor) seizures.
- It is not known if PEGANONE is safe or effective in children younger than 1 year old.
- Who should not take PEGANONE?
- Do not take PEGANONE if you have:
- What should I tell my healthcare provider before taking PEGANONE?
- Before you take PEGANONE, tell your healthcare provider if you:
- Have or have had depression, mood problems, or suicidal thoughts or behavior
- Have any other medical conditions
- Are pregnant or plan to become pregnant. PEGANONE may harm your unborn baby and cause birth defects. Birth defects may occur even in children born to women who are not taking any medicines and do not have other risk factors.
- Tell your healthcare provider right away if you become pregnant while taking PEGANONE. You and your healthcare provider should decide if you will take PEGANONE while you are pregnant.
- If you become pregnant while taking PEGANONE, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- Are breastfeeding or plan to breastfeed. PEGANONE can pass into breast milk.
- You and your healthcare provider should decide if you will take PEGANONE or breast feed. You should not do both. Talk to your healthcare provider about the best way to feed your baby if you take PEGANONE.
- Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
- Taking PEGANONE with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
- Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
- Especially tell your healthcare provider if you take medicines that affect blood clotting.
- How should I take PEGANONE?
- Take PEGANONE exactly as your healthcare provider tells you. Your healthcare provider will tell you how much PEGANONE to take.
- Your healthcare provider may change your dose. Do not change your dose of PEGANONE without talking to your healthcare provider.
- Do not stop taking PEGANONE without first talking to your healthcare provider. Stopping PEGANONE suddenly can cause serious problems.
- Take PEGANONE after eating, and space doses out evenly.
- If you take too much PEGANONE, call your healthcare provider or local Poison Control Center right away.
- What are the possible side effects of PEGANONE?
- See “What is the most important information I should know about PEGANONE?”.
- PEGANONE may cause other rare, but serious side effects. These include:
- Blood problems – symptoms may include: not feeling well, sore throat, fever, bruising easily, red or purple spots on your body, nose bleed
- Liver problems – symptoms may include: yellowing of your skin or the whites of your eyes (jaundice), dark urine, nausea or vomiting, loss of appetite, pain on the right side of your stomach, bruising easily.
- Swollen glands (enlarged lymph nodes)
- Lupus – symptoms may include: a rash on your cheeks or other parts of your body, sensitivity to the sun, new joint or muscle pains, chest pain or shortness of breath, swelling of your feet, ankles, and legs.
- Serious rash – symptoms may include: skin rash, hives, sore throat, sores in your mouth, your skin blisters and peels, swelling of your face,eyes, lips, tongue, or throat.
- Call your healthcare provider right away if you have any of the symptoms listed above.
- The most common side effects of PEGANONE include:
- These are not all the possible side effects of PEGANONE. For more information, ask your healthcare provider or pharmacist.
- Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
- Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- How should I store PEGANONE?
- Store at 20-25°C (68-77°F).
- Keep PEGANONE in a tightly closed container, and keep PEGANONE out of the light.
- Keep PEGANONE and all medicines out of the reach of children.
- General Information about PEGANONE
- Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PEGANONE for a condition for which it was not prescribed. Do not give PEGANONE to other people, even if they have the same symptoms that you have. It may harm them.
- This Medication Guide summarizes the most important information about PEGANONE. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about PEGANONE that is written for health professionals.
- For more information, go to www.recordatirarediseases.com or call 1-888-575-8344.
Precautions with Alcohol
- Alcohol-Ethotoin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PEGANONE ®[1]
Look-Alike Drug Names
There is limited information regarding Ethotoin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Ethotoin |Label Name=Peganone 03.jpg
}}
{{#subobject:
|Label Page=Ethotoin |Label Name=DailyMed - PEGANONE - ethotoin tablet .png
}}

