Nevirapine clinical studies
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Studies
Adult Patients
The clinical efficacy of VIRAMUNE XR is based on 96-week data from an ongoing, randomized, double-blind, double-dummy Phase 3 trial (Trial 1100.1486, VERxVE) in treatment-naïve subjects and on 48-week data in an ongoing, randomized, open-label trial in subjects who switched from immediate-release VIRAMUNE tablets administered twice daily to VIRAMUNE XR tablets administered once daily (Trial 1100.1526, TRANxITION).
Treatment-naïve Subjects
Trial 1100.1486 (VERxVE) is a Phase 3 trial in which treatment-naïve subjects received immediate-release VIRAMUNE 200 mg once daily for 14 days and then were randomized to receive either immediate-release VIRAMUNE 200 mg twice daily or VIRAMUNE XR 400 mg once daily. All subjects received tenofovir + emtricitabine as background therapy. Randomization was stratified by screening HIV-1 RNA level (less than or equal to 100,000 copies per mL and greater than 100,000 copies per mL). Subject demographic and baseline disease characteristics were balanced between the two treatment groups. With respect to demographics: 85% of the subjects were male, 75% were white, 20% were black, and approximately 29% were from North America. With respect to baseline disease characteristics: mean viral load was 4.7 log10copies per mL, mean CD4+ cell count was 228 cells/mm3 and 73% of subjects had clade B HIV-1 subtype. Approximately two-thirds of the subjects had a baseline HIV-RNA level of less than or equal to 100,000 copies per mL.
Table 6 describes week 96 outcomes in the Trial 1100.1486 (VERxVE). These outcomes include all subjects who were randomized after the 14 day lead-in with immediate-release VIRAMUNE and received at least one dose of blinded study medication.
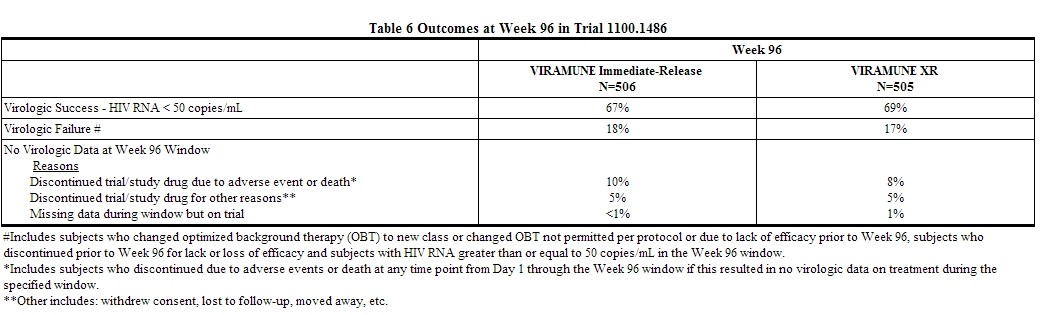 |
At 96 weeks, mean change from baseline in CD4+ cell count adjusting for baseline HIV-1 viral load stratum was 222 cells/mm3 and 244 cells/mm3 for the groups receiving immediate-release VIRAMUNE and VIRAMUNE XR, respectively.
Subjects Switching from Immediate-release VIRAMUNE to VIRAMUNE XR
Trial 1100.1526 (TRANxITION) is a Phase 3 trial to evaluate safety and antiviral activity of switching from immediate-release VIRAMUNE to VIRAMUNE XR. In this open-label trial, 443 subjects already on an antiviral regimen containing immediate-release VIRAMUNE 200 mg twice daily with HIV-1 RNA less than 50 copies per mL were randomized in a 2:1 ratio to VIRAMUNE XR 400 mg once daily or immediate-release VIRAMUNE 200 mg twice daily. Approximately half of the subjects had tenofovir+emtricitabine as their background therapy, with the remaining subjects receiving abacavir sulfate+lamivudine or zidovudine+lamivudine. Approximately half of the subjects had at least 3 years of exposure to immediate-release VIRAMUNE prior to entering the trial.
At 48 weeks after randomization in Trial 1100.1526, 91% of subjects receiving immediate-release VIRAMUNE 200 mg twice daily and 93% of subjects receiving VIRAMUNE XR 400 mg once daily continued to have HIV-1 RNA less than 50 copies per mL.
Pediatric Patients
Trial 1100.1518 was an open-label, multiple-dose, non-randomized, crossover trial performed in 85 HIV-1 infected pediatric subjects 3 to less than 18 years of age who had received at least 18 weeks of immediate-release VIRAMUNE and had plasma HIV-1 RNA less than 50 copies per mL prior to trial enrollment. Subjects were stratified according to age (3 to less than 6 years, 6 to less than 12 years, and 12 to less than 18 years). Following a 10-day period with immediate-release VIRAMUNE, subjects were treated with VIRAMUNE XR tablets once daily in combination with other antiretrovirals for 10 days, after which steady-state pharmacokinetic parameters were determined. Forty of the 80 subjects who completed the initial part of the study were enrolled in an optional extension phase of the trial which evaluated the safety and antiviral activity of VIRAMUNE XR through a minimum of 24 weeks of treatment. Zidovudine or stavudine plus lamivudine were the most commonly used background therapies in subjects who entered the optional extension phase.
Baseline demographics included: 55% of the subjects were female, 93% were black, 7% were white, and approximately 84% were from Africa. Subjects had a median baseline CD4+ cell count of 925 cells/mm3 (range 207 to 2057 cells/mm3).
Of the 40 subjects who entered the treatment extension phase, 39 completed at least 24 weeks of treatment and one subject discontinued prematurely due to an adverse reaction. After 24 weeks or more of treatment with VIRAMUNE XR, all 39 subjects continued to have plasma HIV-1 RNA less than 50 copies per mL. Median CD4+cell counts for the 3 to less than 6 year, 6 to less than 12 year, and 12 to less than 18 year age groups were 1113 cells/mm3, 853 cells/mm3, and 682 cells/mm3, respectively. These CD4+ cell counts were similar to those observed at baseline.[1]
References
- ↑ "VIRAMUNE (NEVIRAPINE) TABLET, EXTENDED RELEASE [BOEHRINGER INGELHEIM PHARMACEUTICALS, INC.]". Retrieved 10 January 2014.
Adapted from the FDA Package Insert.